��Ŀ����
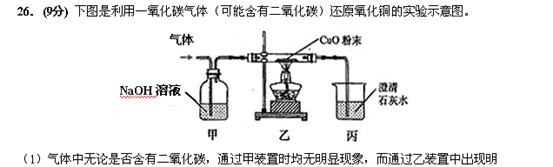 �Ե������������� ��
�Ե������������� ��
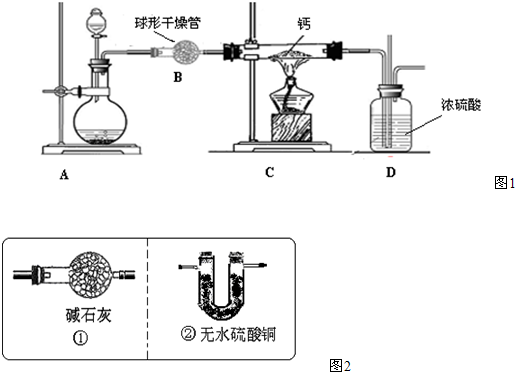
��2�����пɹ۲쵽��ʵ�������� ��
��3����װ�ô��ڵ���Ҫ������ ��
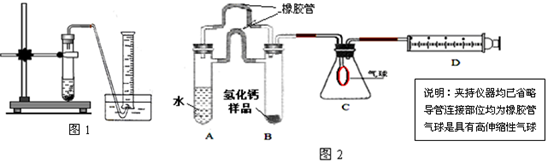
��4��ʵ����Ϻ��������ʵ���֤ʵ��װ��������������Һ�Ƿ����ն�����̼�����ʣ�
| ����֤��ʵ�� | ʵ�鲽�� | ʵ������ | ��ѧ����ʽ |
| ��һ��ԭ������������̼����װ��������������Һû���� | ȡ������Һ���Թ��У��μ�ϡ���� | �����ݷų� | NaOH+HCl=NaCl+H2O |
| ������ԭ�����к�������̼����װ��������������Һ���ֱ��� | ��ȡ������Һ���Թ��У� �μӹ��� ��Һ�� �ھ��ã�ȡ�ϲ���Һ����һ�Թ��У��μӷ�̪��Һ�� | �� �а�ɫ�������ɣ� �� | �� |
| ������ԭ�����к�������̼����װ��������������Һ��ȫ���� | ͬ��������ʵ�鲽�� | �� �а�ɫ�������ɣ� �� | �� |
| ��˼ | ��Ϊ������Ҳ���ж�����̼�������������Ʊ����ܷ�ȫƷ��ѧ���� �ú��벻�ˣ����档 |
��5����ԭ�����к�������̼���Ҽ�װ��������������Һ���ֱ��ʣ�Ҫ��ȥ��װ���е����ʣ�ʹ����ش���������������Һ��Ӧѡ�õ��Լ��� ��
(1) ��ɫ��ĩ��� ��2��������Һ����� ��3��û��β������װ��
��4��
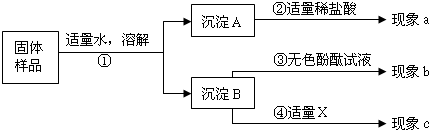
| ʵ�鲽�� | ʵ������ | ��ѧ����ʽ | |
| ������ | �� �Ȼ��� | �ڱ�� | ��CaCl2+ Na2CO3 ==CaCO3��+ 2NaCl |
| ������ | �ڲ���ɫ |
��5������������Һ