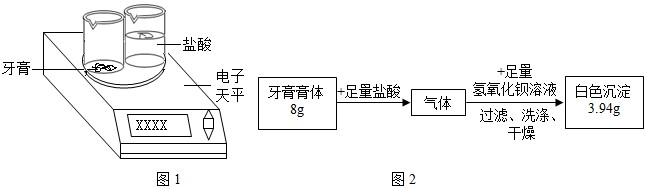
��Ŀ����
ij�о���ѧϰС���ij����۽���������̽����
���������ϡ�������Ļ�ѧ�ɷ���Ҫ�ǣ�̼��Ƽ�����̼��þ�Ϳǽǵ���(�����ʵ�һ��)��
�ڿǽǵ��ײ�����ˮ������Ũ������Ⱥ����ɫ��
�ۼ��������ʵ���ǡ����Ƿۡ�����Ҫ�ɷ־���̼��ơ�
���������ۡ����������������ǽǵ������������������ ���� ���л����������������
�ڼ�������е�̼��ƺ���Ҫ��������۴�
��ʵ��̽�����о���ѧϰС���������������������
����һ ��������۵����
ȡ�����������Ʒ���Թ��У� �����۲쵽 ������������������ۡ�
������ �����������е�̼��ƺ���
������������������ͼ��ʾʵ��װ�á�ijѧ����סC�����ܣ���A��װ������ˮ��ȡ���ϲ����ӣ�����������A��ˮ����ȫ�����£�����Ϊ��װ���Ƿ�©���� ��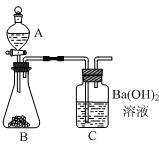
�����ȷ��ȡ6.000 g�������Ʒװ������B�У���A��װ��ϡ���ᡣ
�������B���������Ʒ�еμ�������ϡ���ᡣ������B��C�пɹ۲쵽������Ϊ �� ��
���������ȫ��Ӧ��C�е���Һ�� �� ������Ƶð�ɫ���������Ϊ11.820g�����������Ʒ��̼��Ƶ���������Ϊ100%��
��ʵ�鷴˼����ô˲ⶨ��������ܵ�ԭ���� ������������ţ�
����Ʒ�к���̼��þ
������δ�μ�����
��CO2�����ٶ�̫�쵼��δ��Ba(OH)2��ȫ����
��װ��B��ˮ������HCl�Ƚ���װ��C��
����չ���졿��ͬѧ����ڷ������в���Ҫװ��C��ֻҪ�õ�����ƽȷ����װ��A��B��Ӧǰ����������Ϳ��Եõ��������Ʒ��̼��Ƶĺ���������Ϊ�Ƿ���� ����˵������ ��
���������ۡ� �л�
��ʵ��̽��������Ũ���Ტ���� ���ɫ ��©�� B�й����ܽⲢ�������ݣ� C�в������� ���� ϴ��
��ʵ�鷴˼����
����չ���졿������ ���������غ㶨�ɣ�װ��A��B��Ӧǰ������������̼����Լ�̼��þ�����ᷴӦ���ɵ�CO2��������������Ϊ��֪��̼��ƺ�̼��þ���������������������Ʒ��̼��Ƶ�������������
����������������������ۡ��ǽǵ������ڵ����ʣ�����������������л������һ�������м���Ũ���Ტ���������Ϊ��ɫ��˵���пǽǵ��ף�Ϊ�����飻��������ͼ��ʾ����סC�����ܷ�Һ©���е�ˮ������ȫ����˵��װ�ò�©�������������ã������װ��B�еķ�ӦΪ�����̼��Ʒ������ֽⷴӦ�����Ȼ��ơ�ˮ�Ͷ�����̼�ķ�Ӧ������Ϊ������ð����A�����������Ͷ�����̼��Ӧ����̼�ᱵ�����������а�ɫ�������ɣ��������Һ�������ˡ�ϴ�ӡ������õ�������̼�ᱵ���������������������̼������������ͨ��������̼�������������̼��Ƶ���������ʵ�鷴˼�������Ǻ���̼��þ���µģ���λ������̼��þ�����Ķ�����̼�϶ࣻ����չ���졿�����У���Ϊ���������غ㶨�ɣ�װ��A��B��Ӧǰ������������̼����Լ�̼��þ�����ᷴӦ���ɵ�CO2��������������Ϊ��֪��̼��ƺ�̼��þ���������������������Ʒ��̼��Ƶ�������������
���㣺̽������ɷ�
����������һ���dz����͵�̽���⣬��Ŀ����֪ʶ��û��ѧ������Ϊİ���������ص㿼��ķ�Ӧ��˼�룬װ�õȣ�����������Ŀ��Ҫ���£���ϸ���⼴�ɡ�

 ϰ�⾫ѡϵ�д�
ϰ�⾫ѡϵ�д���1�����룺ά����C���ܾ������ԣ�
��2��ʵ�鷽����
�ṩ�Լ���ά����CƬ������ˮ����ɫʯ����Һ����ɫ��̪��Һ��ϡ���ᡢ����������Һ���Ȼ�����Һ��pH��ֽ��
���������Լ����������������Ƴ�һ��ʵ�鷽������д��ʵ��������Ƴ����ַ�������4�֣�
| ʵ������ | ʵ������ |
| ����1��______ | ______ |
| ����2��______ | ______ |
A������þ B���Ȼ��� C���������� D������ͭ
��4��ijͬѧ���뵽���������߲ˡ�ˮ���к��зḻ��ά����C������ʱ�䳤���Ƿ��ά����C�ĺ�������Ӱ�죮�����������ʵ�鷽����
��������������ͷ���һ�ܵ��������ֱ��飬��ɴ����֭Һ���������ձ��У�
��ȡ��֧ʢ��2ml��ɫ��ĵ�����Һ���Թܣ��ֱ�μ���������֭Һ���ӱ���ֱ����ɫ�պ���ʧ����¼�������£�
| ֭Һ | ������������֭Һ | ����һ�ܵ���������֭Һ |
| ���� | 12 | 20 |
�������������������У�����Ϊά����C�����ߵ���______��
������еõ�����ʾ��______��
��1�����룺ά����C���ܾ������ԣ�
��2��ʵ�鷽����
�ṩ�Լ���ά����CƬ������ˮ����ɫʯ����Һ����ɫ��̪��Һ��ϡ���ᡢ����������Һ���Ȼ�����Һ��pH��ֽ��
���������Լ����������������Ƴ�һ��ʵ�鷽������д��ʵ��������Ƴ����ַ�������4�֣�
| ʵ������ | ʵ������ |
| ����1��______ | ______ |
| ����2��______ | ______ |
A������þ B���Ȼ��� C���������� D������ͭ
��4��ijͬѧ���뵽���������߲ˡ�ˮ���к��зḻ��ά����C������ʱ�䳤���Ƿ��ά����C�ĺ�������Ӱ�죮�����������ʵ�鷽����
��������������ͷ���һ�ܵ��������ֱ��飬��ɴ����֭Һ���������ձ��У�
��ȡ��֧ʢ��2ml��ɫ��ĵ�����Һ���Թܣ��ֱ�μ���������֭Һ���ӱ���ֱ����ɫ�պ���ʧ����¼�������£�
| ֭Һ | ������������֭Һ | ����һ�ܵ���������֭Һ |
| ���� | 12 | 20 |
�������������������У�����Ϊά����C�����ߵ���______��
������еõ�����ʾ��______��