��Ŀ����
����Ŀ��ij������ˮ��ˮԴ����һ������ˮ�⣬ij��ѧ��ȤС�鿪չ��һ�ξ���ˮ��ʵ�������ش�����������

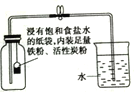
(1)����ȡˮ�����й��ˡ�������ֽ����ͼ�ṩ�������⣬����Ҫ��һ�ֲ���������_________��
(2)����Һ�м������̿��������________�Գ�����ζ���ٴι��ˡ�
(3)Ϊ�˼���������Һ�Ƿ�ΪӲˮ��ȡ������__________�����ݲ�����ɫ��״�����ĭ�Ķ��ٽ����жϡ�
(4)����ˮ�����������г���Һ�������������ˮ�������·�Ӧ��
Cl2+H2O=HCl+HClO(������)��������ȶ��ֽ���2HClO=2HCl+O2��������ȤС���С��ͬѧ�þ��ú������ˮ������������Һ���а�ɫ�������֡�����Ͳ���������ԭ��_______________________(�û�ѧ����ʽ��ʾ)��
(5)����ˮ�������䰮ˮ��Դ����ÿ������Ӧ�������κ������������������ڱ���ˮ��Դ����_______(���ֺ�)��
A.���ʺ�ũҩ�Ĺ���ʹ�� B.��ҵ��ˮ���������ŷ�
C.ʵ���ҵķ�Һ��������ֱ���ŷ� D.ȼú�м���ʯ��ʯ��Ϊ����������������ˮԴ����Ⱦ
���𰸡� ������ ���� ����ˮ HCl+AgNO3=AgCl��+HNO3 B D
����������1������װ��Ҫ����̨��©������ֽ���ձ��������������������
��2������̿���������ԣ���������ɫ�غ���ζ��
��3��������Ӳˮ�÷���ˮ������ĭ�ม���ٵ�ˮΪ��ˮ����֮ΪӲˮ��
��4������ˮ�е�ϡ��������������Ӧ�����Ȼ������������ᣬ��Ӧ����ʽΪHCl+AgNO3=AgCl��+HNO3��
��5��A.���ʺ�ũҩ�Ĺ���ʹ�û�ʹˮ����Ⱦ������
B.��ҵ��ˮ���������ŷſ��Լ���ˮ����Ⱦ����ȷ��
C.ʵ���ҵķ�Һ��������ֱ���ŷŻ����ˮ����Ⱦ������
D.ȼú�м���ʯ��ʯ��Ϊ����������������ˮԴ����Ⱦ����ȷ����ѡBD��

 53���ò�ϵ�д�
53���ò�ϵ�д�����Ŀ��Ϊ̽��CO2��NaOH��Һ�����ķ�Ӧ��ij��ȤС�鳢���ò�ͬ�ķ�ʽ����ʵ����
������������
��.20��ʱ������������ˮ�е��ܽ�ȼ��±���
���� | Na2CO3 | NaHCO3 | Ca(OH)2 | Ba(OH)2 |
�ܽ��/g | 21.5 | 9.6 | 0.165 | 3.89 |
��.��ʵ����������Na2CO3��Һ��NaHCO3��Һ��pH�ֱ�ԼΪ11.0��8.5��
��ʵ��̽����
(1)ʵ��һ��С��ȡһ����CO2�Ŀ�Ȫˮƿ������һ������ˮ������š��ƿ������������ƿ�ӱ����С����ȡһ��ͬ�ij���CO2�Ŀ�Ȫˮƿ�������м�����ˮ�������NaOH��Һ������š��ƿ���������õ���ҺX����ʱ�۲쵽��������_________________________________��ʵ������С�������ֻ��Ȫˮƿ���Ա�ʵ���Ŀ����_________________________________��
(2)ʵ�����Ϊ����CO2��NaOH��Һ��Ӧ�IJ�����С��ȡʵ��һ������ҺX�����������еμ�BaCl2��Һ���а�ɫ�����������÷�Ӧ�Ļ�ѧ����ʽΪ_____________________________��ʵ���в��˽�BaCl2��Һ����CaCl2��Һ��ԭ����_____________________________________��
(3)ʵ������С��ȡʵ��һ������ҺX�����������м��������BaCl2��Һ������������ȡ�ϲ���Һ������1�η�̪��Һ��������Һ��____ɫ��֤����ҺX����NaOHʣ����ʵ������С��û��ֱ����������ҺX�е����̪��Һ��������___________________________________��
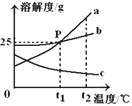
(4)ʵ��������ȤС�齫CO2����ͨ��һ��Ũ��һ������NaOH��Һ���������ֻ�ʵ�鼼���ⶨ��Ӧ��������Һ��pH���¶ȱ仯�������ͼ1��ͼ2��ʾ��
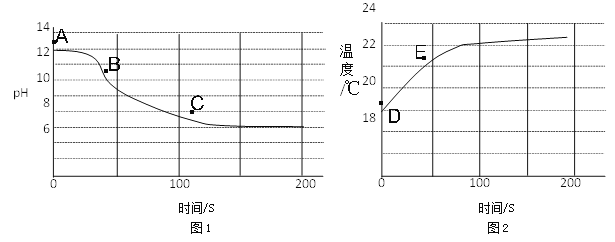
ͼ1����BC�η�����Ӧ�Ļ�ѧ����ʽΪ____________________________________��
ͼ2����DE���¶ȱ仯��ԭ����__________________________________________��
����˼������
(5)ʵ���CO2���١�NaOH������Na2CO3���ɵ����ʵı仯���Լ�___________ת�����ӽǶ�ά��̽��CO2��NaOH�����˷�Ӧ�������������ԵĻ�ѧ��Ӧ������ͨ���ִ������ֶν������ݲⶨ��ʵ�ַ�Ӧ���̵������ӻ�����
����Ŀ��ʵ��С��ͬѧͨ������ʵ���о�������̼�����ʡ�
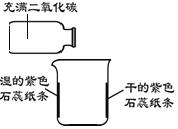
| �����ձ��ڱ������ϸɵĺ���ˮ��ʪ����ɫʯ��ֽ�� �ڽ�����������̼�ļ���ƿ�������ձ��Ϸ���б | һ��ʱ���ʪ����ɫʯ��ֽ����Ϊ��ɫ |
(1)�ձ��ڱ������ɵ���ɫʯ��ֽ����Ŀ����__________��
(2)��ʵ�鷢����Ӧ�Ļ�ѧ����ʽΪ__________��