��Ŀ����
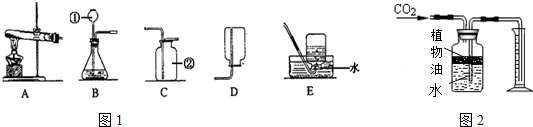
��ͼ�ף�A-F��Ϊʵ���ҳ��õ�ʵ��װ�ã��ش����⣺

��1��д�����б�Ţ�����������______��
��2��ʵ�������ü���̼�����ƣ�NaHCO3�����壨����Ϊ̼���ơ�������̼��ˮ������ȡCO2�÷�Ӧ�Ļ�ѧ����ʽ______�����ô˷�����ȡһƿ�����CO2Ӧѡ�õ�װ��Ϊ��������ĸ��ţ�______��
��3��ͼ����ʾװ�ÿ������������ɵ�CO2�������������ˮ���Ϸ�һ��ֲ���͵�Ŀ����______��ֲ�����Ϸ�ԭ�п�����ʵ����______����С����ޡ�������Ӱ�죮

��1��д�����б�Ţ�����������______��
��2��ʵ�������ü���̼�����ƣ�NaHCO3�����壨����Ϊ̼���ơ�������̼��ˮ������ȡCO2�÷�Ӧ�Ļ�ѧ����ʽ______�����ô˷�����ȡһƿ�����CO2Ӧѡ�õ�װ��Ϊ��������ĸ��ţ�______��
��3��ͼ����ʾװ�ÿ������������ɵ�CO2�������������ˮ���Ϸ�һ��ֲ���͵�Ŀ����______��ֲ�����Ϸ�ԭ�п�����ʵ����______����С����ޡ�������Ӱ�죮
��1����ͼ��֪Ϊ����ƿ��
��2������̼�����ƣ�NaHCO3�����壨����Ϊ̼���ơ�������̼��ˮ������ȡCO2�÷�Ӧ�Ļ�ѧ����ʽΪ��2NaHCO3
Na2CO3+CO2��+H2O�����ڶ�����̼���ܶȴ��ڿ������ռ�������̼������������ſ�������������̼���������Ũ�������ʿ���Fװ���ռ�����Ķ�����̼���壻
��3��Ҫ����������̼�����ֻ������ˮ��������Ͳ����ˮ������Ӷ��������̼�������������̼������ˮ������Ҫ��ˮ���Ϸ���һ��ֲ���ͣ�ֲ�����Ϸ�ԭ�еĿ��������ܽ���ˮ�У��������Ϸ��Ŀ�����ʵ����û��Ӱ�죮
�ʴ�Ϊ����1������ƿ��
��2��2NaHCO3
Na2CO3+CO2��+H2O��F��
��3����ֹ������̼����ˮ���ޣ�
��2������̼�����ƣ�NaHCO3�����壨����Ϊ̼���ơ�������̼��ˮ������ȡCO2�÷�Ӧ�Ļ�ѧ����ʽΪ��2NaHCO3
| ||
��3��Ҫ����������̼�����ֻ������ˮ��������Ͳ����ˮ������Ӷ��������̼�������������̼������ˮ������Ҫ��ˮ���Ϸ���һ��ֲ���ͣ�ֲ�����Ϸ�ԭ�еĿ��������ܽ���ˮ�У��������Ϸ��Ŀ�����ʵ����û��Ӱ�죮
�ʴ�Ϊ����1������ƿ��
��2��2NaHCO3
| ||
��3����ֹ������̼����ˮ���ޣ�

��ϰ��ϵ�д�
 ��ѧ�̸̳����¿α�ϵ�д�
��ѧ�̸̳����¿α�ϵ�д� Сѧ��ʱ��ѵϵ�д�
Сѧ��ʱ��ѵϵ�д�
�����Ŀ