��Ŀ����
ijУ��ѧ��ȤС��ͬѧ���֣�����ʹ�õ���ˮ���ײ���һ��ˮ����ˮ������Ҫ�ɷ���̼��ƺ�������þ������Ϊ�˲ⶨˮ����̼��Ƶĺ�������������������Ϊ10����������뵽10gˮ���У�����CO2����������ͼ��ʾ�� 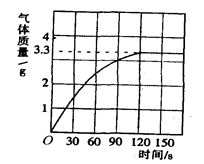
(1) ��ͼ�п��Կ�����10gˮ�������ᷴӦ���ɵĶ�����̼�����________ g��
(2) ˮ����̼��Ƶ�������________ g��
(3) ��ȥˮ����̼���������Ҫ��������Ϊ10���������������________ g (���������һλС��)��
��4��д���뱾ʵ���йصĻ�ѧ����ʽ
1��_______________________________________________________________
2��_______________________________________________________________
��1��3.3 ��1�֣� ��2��7.5 ��2�֣� ��3��86.2g��2�֣�
1��CaCO3 + 2HCl ="=" CaCl2 + H2O + CO2�� ��2�֣�
2��Mg��OH��2 + 2HCl ="=" MgCl2 + 2H2O
����

��ϰ��ϵ�д�
�����Ŀ
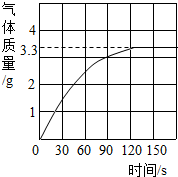 ��2012?������һģ��ijУ��ѧ��ȤС��ͬѧ���֣�����ʹ�õ���ˮ���ײ���һ��ˮ����ˮ������Ҫ�ɷ���̼��ƺ�������þ������Ϊ�˲ⶨˮ����̼��Ƶĺ�������������������Ϊ10%��������뵽10gˮ���У�����CO2����������ͼ��ʾ��
��2012?������һģ��ijУ��ѧ��ȤС��ͬѧ���֣�����ʹ�õ���ˮ���ײ���һ��ˮ����ˮ������Ҫ�ɷ���̼��ƺ�������þ������Ϊ�˲ⶨˮ����̼��Ƶĺ�������������������Ϊ10%��������뵽10gˮ���У�����CO2����������ͼ��ʾ�� 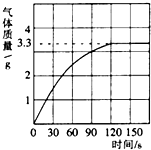 ijУ��ѧ��ȤС��ͬѧ��ʵ��ⶨһ�����ʯ��̼��Ƶ��������������dz�ȡ�Ĵ���ʯ����Ϊ12.5g���������������ϡ�����У���������������ͼ��ʾ������ô���ʯ��̼��Ƶ�����������������ʯ�г�̼���֮����������ʲ������ᷢ����Ӧ��
ijУ��ѧ��ȤС��ͬѧ��ʵ��ⶨһ�����ʯ��̼��Ƶ��������������dz�ȡ�Ĵ���ʯ����Ϊ12.5g���������������ϡ�����У���������������ͼ��ʾ������ô���ʯ��̼��Ƶ�����������������ʯ�г�̼���֮����������ʲ������ᷢ����Ӧ��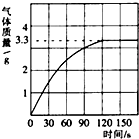 ijУ��ѧ��ȤС��ͬѧ���֣�����ʹ�õ���ˮ���ײ���һ��ˮ����ˮ������Ҫ�ɷ���̼��ƺ�������þ������Ϊ�˲ⶨˮ����̼��Ƶĺ�������������������Ϊ10%��������뵽12.5gˮ������CO2����������ͼ��ʾ��
ijУ��ѧ��ȤС��ͬѧ���֣�����ʹ�õ���ˮ���ײ���һ��ˮ����ˮ������Ҫ�ɷ���̼��ƺ�������þ������Ϊ�˲ⶨˮ����̼��Ƶĺ�������������������Ϊ10%��������뵽12.5gˮ������CO2����������ͼ��ʾ��