��Ŀ����
�ҹ��ֵ��������������Ҫԭ�����ɾ������ȼ�պ����ú��ijЩ�����������������ŷŵĶ����������徭��һϵ�л�ѧ��Ӧ���γɵģ�ij��ѧ��ȤС����̽��ijһ������������ȣ�̽���������£�
[��������]��ˮ��pH����Ϊ��______5.6��������ڡ�����С�ڡ��������ڡ���
[��ƺ�ʵ��]Ϊ�ⶨij��ˮ�Ƿ��������꣬Ӧѡ��______������ţ�
A����ɫʯ����ҺB����ɫ��̪��ҺC��pH��ֽ
����ͬѧ����������ʵ�飺
��ͬѧ��ȡpH��ֽ���ڲ���Ƭ�ϣ��ò�����պȡ��ˮ�ε�pH��ֽ�ϣ�����ֽ��ʾ����ɫ�����ɫ���Ƚϣ�
��ͬѧ��ȡpH��ֽ���ڲ���Ƭ�ϣ���������ˮ��pH��ֽ��ʪ��Ȼ���ò�����պȡ��ˮմ��pH��ֽ�ϣ�����ֽ��ʾ����ɫ�����ɫ���Ƚϣ�
[���������]��λͬѧ�в�����ȷ����______ͬѧ��ָ����һλͬѧ�IJ�������______������������ɵĽ����______��
[�����뷴˼]������ɵ�Σ���ܴ���______����дһ�ֺ����𰸣�����ֹ����Ĵ�ʩ��______����дһ�ֺ����𰸣���
[��������]��ˮ��pH����Ϊ��______5.6��������ڡ�����С�ڡ��������ڡ���
[��ƺ�ʵ��]Ϊ�ⶨij��ˮ�Ƿ��������꣬Ӧѡ��______������ţ�
A����ɫʯ����ҺB����ɫ��̪��ҺC��pH��ֽ
����ͬѧ����������ʵ�飺
��ͬѧ��ȡpH��ֽ���ڲ���Ƭ�ϣ��ò�����պȡ��ˮ�ε�pH��ֽ�ϣ�����ֽ��ʾ����ɫ�����ɫ���Ƚϣ�
��ͬѧ��ȡpH��ֽ���ڲ���Ƭ�ϣ���������ˮ��pH��ֽ��ʪ��Ȼ���ò�����պȡ��ˮմ��pH��ֽ�ϣ�����ֽ��ʾ����ɫ�����ɫ���Ƚϣ�
[���������]��λͬѧ�в�����ȷ����______ͬѧ��ָ����һλͬѧ�IJ�������______������������ɵĽ����______��
[�����뷴˼]������ɵ�Σ���ܴ���______����дһ�ֺ����𰸣�����ֹ����Ĵ�ʩ��______����дһ�ֺ����𰸣���
��Ϊ��������ˮ�У��ܽ���CO2�����Բ�����ˮ��PH���ܵ���5.6����ˮ�Ƿ��������꣬Ҫ�ⶨPH�Ƿ�С��5.6��������PH��ֽ�Ƚ�ȷ����ͬѧ������ȷ����ͬѧ�����Ĵ���֮������������ˮ��pH��ֽ��ʪ���Ǿ��൱�ڰ���ˮϡ���ˣ�������ʹ�ǽ����������ϣ���������ɰ���ͻ�ɰש������Ӳ��ˮ���ܽ⣬���ֿն����ѷ죬����ǿ�Ƚ��ͣ��Ӷ�������������ɵ��������ữ����ȼú������������ʹ�������Դ�ȶ��ܷ�ֹ���꣮
�ʴ𣺵��ڣ�C����ͬѧ����������ˮ��pH��ֽ��ʪ���൱�ڰ���ˮϡ���ˣ�����ɵ��������ữ����ȼú������������
�ʴ𣺵��ڣ�C����ͬѧ����������ˮ��pH��ֽ��ʪ���൱�ڰ���ˮϡ���ˣ�����ɵ��������ữ����ȼú������������

��ϰ��ϵ�д�
 ���ƽ̸�������ѡ����ĩ���100��ϵ�д�
���ƽ̸�������ѡ����ĩ���100��ϵ�д�
�����Ŀ
������pHС��5.6�Ľ�ˮ���ҹ��ֵ�������������Ҫ���ɾ������ȼ�պ����ú�Լ�ijЩ�����������������ŷŵĶ����������壬����һϵ�л�ѧ��Ӧ���γɵģ�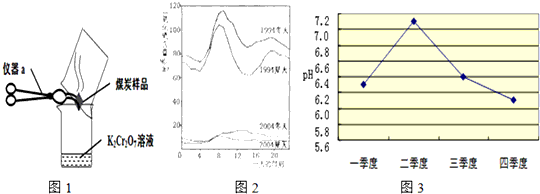
��1����������������ȼ�գ��۲쵽��������________���÷�Ӧ�Ļ�ѧ����ʽΪ________��
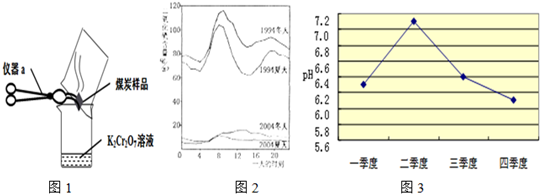
��2����λͬѧΪ��̽��ľ̿���Ƿ���������Ԫ�أ������������ͼ1��ʾʵ����вⶨ����д��ͼ������a�����ƣ�________�����Dz������Ϻ��֪��������������ʹK2Cr2O7��Һ��ɫ���ɳȺ�ɫ�����ɫ������Ӧ����ʽΪ����3SO2+K2Cr2O7+H2SO4�TK2SO4+ +H2O����Ȼ����ѧ����ʽ����һ�����ﲻ������������ѧ֪ʶ�Ʋ��仯ѧʽ��________����ӦǰK2Cr2O7��CrԪ�ػ��ϼ�Ϊ________�ۣ�
+H2O����Ȼ����ѧ����ʽ����һ�����ﲻ������������ѧ֪ʶ�Ʋ��仯ѧʽ��________����ӦǰK2Cr2O7��CrԪ�ػ��ϼ�Ϊ________�ۣ�
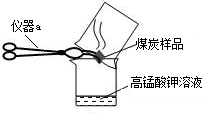
��3����ͼ2��1994���2004��ij���е�һ�����ʱ�̲�Ŀ����ж�������ĺ���������˵������ȷ����________
A��������ʾ��������еĶ����������������
B��������ʾ��1994��һ���д�Լ8�����Ҷ�������ĺ����ϸ�
C����������ĺ�����10��併�͵�ԭ������Ǽ�ǿ��ȼ�ϵ�����Ϳ����˶���������ŷ�
D������������Ⱦ����Ҫ��Դ�������ŷŵ�β����������Ⱦ�ķ����ǽ�ֹʹ������
��4�����о�����Σ���Ĺ����У��ⶨ�õ�����ˮ��pH��ʵ�����________��
ij��ѧ��ȤС��ȡ�ս����������ˮ��ÿ��һ��ʱ���ýϾ��ܵ�pH�Ʋⶨ��pH���������£�
| �ⶨʱ��/���� | 0 | 1 | 2 | 3 |
| pH | 4.73 | 4.62 | 4.56 | 4.55 |
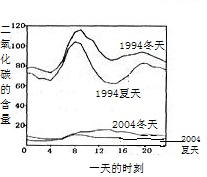
��5��������ɵ�Σ���࣮ܶij��ȤС����鷢��ij�е�ʯ�̵ı����в�ͬ�̶ȵĸ�ʴ���ҽ�20��ĸ�ʴ�ٶȴ����������������Ҫԭ��֮һ�������꣮Ϊ�˼���ʯ�̸�ʴ��������һ�����飺________�������ʹ�����������ữ����ͼ3��ij��ȥ��ij����һ���в�ͬʱ�ں�ˮ��ƽ��pH�仯������ͼ����ȥ���ˮ������ǿ�ļ����ǵ�________���ȣ��ü�����ˮ������ǿ��ԭ������ǣ�����������________��________��
���껹��ʹ�����ữ��Ϊ���к���������������ʹ����ʯ�ҷ�ĩ����������ijɷ������ᣬ��д�����кͷ�Ӧ�Ļ�ѧ����ʽ________��
��6��ijУ��ѧ��ȤС����ѧ���Ļ�ѧ���ʺ��뵽��NaOH��Һ����SO2����Ӧ��ѧ����ʽ���£�2NaOH+SO2�TNa2SO3+H2O����NaOH��Һ����1000L�ѳ�ȥCO2�Ŀ�����Ʒ����Һ����������0.64g����֪��ʱ�������ܶ�ԼΪ1.3g/L����
�ٱ����յ�SO2������________g��
�ڷ�����Ӧ��NaOH�������������ԭ��������Na-23��S-32��O-16��
�ۿ�����SO2��������������������ȷ��0.01%����
 ����Ȼ����ѧ����ʽ�����һ�����ʵĻ�ѧʽӡˢ������������������˽��������һ���ᣬ�������ѧ֪ʶ�Ʋ��仯ѧʽ��
����Ȼ����ѧ����ʽ�����һ�����ʵĻ�ѧʽӡˢ������������������˽��������һ���ᣬ�������ѧ֪ʶ�Ʋ��仯ѧʽ��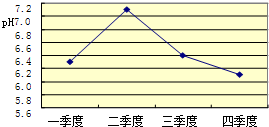
 +H2O����Ȼ����ѧ����ʽ����һ�����ﲻ������������ѧ֪ʶ�Ʋ��仯ѧʽ��
+H2O����Ȼ����ѧ����ʽ����һ�����ﲻ������������ѧ֪ʶ�Ʋ��仯ѧʽ��