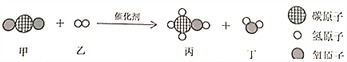
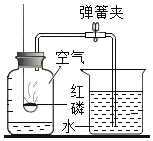
��Ŀ����
����Ŀ���ƵĻ��������ճ����������Ź㷺��Ӧ�á�
��1����������飬����������θ�����ĺ��Ƶ�����______���ѧʽ����
��2��С��ͬѧ���кͷ�Ӧ��̽����Ӧ����������£����Թ�ȡ��������������Һ���μӷ�̪����Һ��______ɫ�������Թ����������ϡ���ᣬ����Һ�ֱ�ɫ����ʱ��Һ��______�ԣ�ȡ��Ӧ�����Һ�����������˰�ɫ���壬���ڰ�ɫ����ɷ֣�ָʾ�����ԣ���С����Ϊ���Ȼ��ƣ�С����Ϊ���������Ȼ��ƺ��������ƵĻ�������Ϊ˭���ж���ȷ______��
��3��ʢ������������Һ���Լ�ƿ���ܷⲻ�ϻ�ʹ�������Ʒ������ʣ��������Ʊ��ʵ�ԭ����______���û�ѧ����ʽ��ʾ����������ҺΪ���ֱ��ʣ������ʵ��֤��______������д����Ҫ�IJ��衢����
��4����һƿ̼���ƺ��������ƹ��������ȡ����Ʒ10g������һ����ϡ������Һǡ����ȫ��Ӧ��ͬʱ������2.2g���塣�Լ���û�������������Ƶ���������______��
���𰸡�NaHCO3 �� ���Ի��� С�����ж�����ȷ�� CO2+2NaOH=Na2CO3+H2O ȡ��Һ�����������еμ������Ȼ�����Һ��������ɫ����̼��ƺ��Ȼ��ƣ�����Һ�еμ���ɫ��̪��Һ������Һ���ɫ��˵�������������� 47%
��������
��1�������г���������θ����������̼�����ƣ���ѧʽΪ��NaHCO3��
��2�����Թ�ȡ��������������Һ���μӷ�̪����Һ���ɫ�������Թ����������ϡ���ᣬϡ������������Ʒ�����Ӧ�����Ȼ��ƺ�ˮ������Һ�ֱ�ɫ��˵���������Ʊ������꣬��Һ���������Ȼ��ƻ��Ȼ��ƺ��Ȼ��⣬�����Һ�����Ժ����ԣ�ȡ��Ӧ�����Һ�����������˰�ɫ���壬��ɫ����ֻ�����Ȼ��ƣ���Ϊһ����ϡ�����������ģ���һ�������������ƴ��ڵ������£���Һ�Ǻ�ɫ�ģ����С�����ж�����ȷ�ģ�
��3��ʢ������������Һ���Լ�ƿ���ܷⲻ�ϻ�ʹ�������Ʒ������ʣ���Ϊ����������Һ�����տ����еĶ�����̼����Ӧ�ķ���ʽΪ��CO2+2NaOH=Na2CO3+H2O��������ҺΪ���ֱ��ʣ�����Һ�е�������̼���ƺ��������ƣ���������ʵ����֤��ȡ��Һ�����������еμ������Ȼ�����Һ��������ɫ����̼��ƺ��Ȼ��ƣ�����Һ�еμ���ɫ��̪��Һ������Һ���ɫ��˵�������������ƣ�
��4���������к���������������Ϊx����̼���Ƶ�����Ϊ10-x��
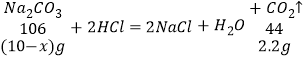
![]()
x=4.7g��
��������������淋���������=![]() %=47%��
%=47%��
����Ʒ���������Ƶ�����Ϊ4.7g��������������Ϊ47%��