��Ŀ����
����ͭ����ͭп�Ͻ𣬾��н�ǿ��е���ܣ���ͷ���ࡰ��ɫ����Ʒ���������������ģ�ij�о���ѧϰС��Ϊ�˲ⶨ��ͭ��ͭ��������������ȡ20g��ͭ��Ʒ�������ձ��У�Ȼ�����ijŨ�ȵ�ϡ����100 g�����������ò��������������ٲ�������Ϊֹ����Ӧ�Ļ�ѧ����ʽΪ��Zn+2HCl�TZnCl2+H2����������ʣ�����ʵ�������Ϊ119.8g������㣺
��1��ʵ������У���������������______����ļ���������______��
��2����ͭп�Ͻ���ͭ������������
��1��ʵ������У���������������______����ļ���������______��
��2����ͭп�Ͻ���ͭ������������
��1�����������غ㶨�ɣ���������������=20g+100g-119.8g=0.2g��
�ʴ�Ϊ��0.2g�������غ㶨�ɣ�
��2���跴Ӧ����п������Ϊx��
Zn+2HCl�TZnCl2+H2��
65 2
x 0.2g
65��2=x��0.2g
��֮�ã�x=6.5g��
��ͭп�Ͻ���ͭ������Ϊ��20g-6.5g=13.5g��
���ͭп�Ͻ���ͭ����������Ϊ��
��100%=67.5%��
�𣺸�ͭп�Ͻ���ͭ����������Ϊ67.5%��
�ʴ�Ϊ��0.2g�������غ㶨�ɣ�
��2���跴Ӧ����п������Ϊx��
Zn+2HCl�TZnCl2+H2��
65 2
x 0.2g
65��2=x��0.2g
��֮�ã�x=6.5g��
��ͭп�Ͻ���ͭ������Ϊ��20g-6.5g=13.5g��
���ͭп�Ͻ���ͭ����������Ϊ��
| 13.5g |
| 20g |
�𣺸�ͭп�Ͻ���ͭ����������Ϊ67.5%��

��ϰ��ϵ�д�
 53���ò�ϵ�д�
53���ò�ϵ�д�
�����Ŀ
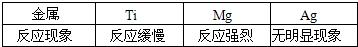
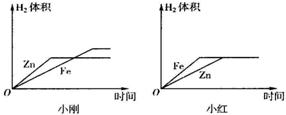
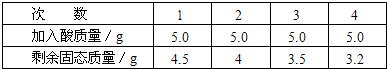
 �û�ѧʽ��ʾ��������
�û�ѧʽ��ʾ��������