��Ŀ����
����Ŀ��(6��)�����ǵ���Ҫ�ɷ���̼���(�����ɷֲ�����ˮҲ�����ᷴӦ)����ѧ��ȤС��Ϊ�˲� ����������̼��Ƶĺ�����������ʵ�飺
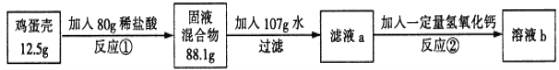
��Ӧ����������������պ÷�Ӧ��һ�룬��Ӧ��ǡ����ȫ��Ӧ����ش��������⣺
��1����Ӧ���Ļ�ѧ����ʽΪ___________________________��
��2��������֪�����г����̼�������(X)�ı���ʽ______________��
��3���ü�������̼��Ƶ���������Ϊ_______��
��4�������������Ƶ�����Ϊ_______��
��5����Һb�����ʵ���������Ϊ_______��
��6����36.5����Ũ��������80g����ϡ�������ˮ������Ϊ_______��
���𰸡���1��CaCO3+2HCl====CaCl2+H2O+CO2��(1��)����2��![]() ����
����![]() ��
��![]() ��
��![]() ������3��80��(1��)����4��7.4g(1��)����5��11.1��(1��)����6��40g(1��)
������3��80��(1��)����4��7.4g(1��)����5��11.1��(1��)����6��40g(1��)
��������
�����������1�������Ǻ���̼��ƣ��������ᷴӦ�����Ȼ��ơ�ˮ�Ͷ�����̼��CaCO3+2HCl==CaCl2+H2O+CO2������2���������غ㶨�ɿɵã���Ӧ�����ɵĶ�����̼������Ϊ��12.5g+80g-88.1g=4.4g,�ٽ�ϻ�ѧ����ʽ�����г����̼�������(X)�ı���ʽ��![]() ����3������2������ɵ�̼��Ƶ�X����Ϊ10g���ʸü�������̼��Ƶ���������Ϊ
����3������2������ɵ�̼��Ƶ�X����Ϊ10g���ʸü�������̼��Ƶ���������Ϊ![]() ��100%=80%����4���ɣ�2����ѧ����ʽ����ɵ÷�Ӧ���Ȼ�������Ϊ7.3g����Ϊ��Ӧ����������������պ÷�Ӧ��һ�룬����Һa���Ȼ��������ҲΪ7.3g���������������Ƶ�����ΪY����ϻ�ѧ����ʽ2HCl+Ca(OH)2==CaCl2+2H2O�����Ȼ�����������ɼ�����������Ƶ�������
��100%=80%����4���ɣ�2����ѧ����ʽ����ɵ÷�Ӧ���Ȼ�������Ϊ7.3g����Ϊ��Ӧ����������������պ÷�Ӧ��һ�룬����Һa���Ȼ��������ҲΪ7.3g���������������Ƶ�����ΪY����ϻ�ѧ����ʽ2HCl+Ca(OH)2==CaCl2+2H2O�����Ȼ�����������ɼ�����������Ƶ�������![]() ��y=7.4g����5����Һb������Ϊ�Ȼ��ƣ���Ӧ���ͷ�Ӧ���������Ȼ��ƣ����ã�2���л�ѧ����ʽ���Լ������Ӧ�����ɵ��Ȼ�������Ϊ11.1g�����ã�4���еĻ�ѧ����ʽ���Ȼ�����������Լ�����Ȼ��Ƶ�����z��
��y=7.4g����5����Һb������Ϊ�Ȼ��ƣ���Ӧ���ͷ�Ӧ���������Ȼ��ƣ����ã�2���л�ѧ����ʽ���Լ������Ӧ�����ɵ��Ȼ�������Ϊ11.1g�����ã�4���еĻ�ѧ����ʽ���Ȼ�����������Լ�����Ȼ��Ƶ�����z��![]() ��z=11.1g���������غ㶨�ɿɵã�������Һ����Ϊ80g+10g-4.4g+107g+7.4g=200g��������Һ����������Ϊ
��z=11.1g���������غ㶨�ɿɵã�������Һ����Ϊ80g+10g-4.4g+107g+7.4g=200g��������Һ����������Ϊ![]() ��100%=11.1%����6����Ӧ������պ÷�Ӧ��һ�룬��80g�����к����Ȼ�������Ϊ7.3g��2=14.6g�������ˮ������Ϊm��������Һϡ��ǰ��������������ɵã���80g-m����36.5%=14.6g������ó�m=40g��
��100%=11.1%����6����Ӧ������պ÷�Ӧ��һ�룬��80g�����к����Ȼ�������Ϊ7.3g��2=14.6g�������ˮ������Ϊm��������Һϡ��ǰ��������������ɵã���80g-m����36.5%=14.6g������ó�m=40g��