��Ŀ����
�������̽��Ҫ�õ����ѧ֪ʶ��
��1���±��г������������е�Ħ���������ڱ�����д����Ħ�����������������
�� ѡ��ᡱ����������Ρ������������
��2����������Ʋ⣬����Ħ�������ܽ�����
��3�������е�̼���Ħ����������ʯ��ʯ���Ʊ���ijѧ�������һ���Ʊ�̼��Ƶ�ʵ�鷽����������ͼΪ��
ʯ��ʯ
��ʯ��
ʯ��ˮ
̼���
��д�������������йط�Ӧ�Ļ�ѧ����ʽ����
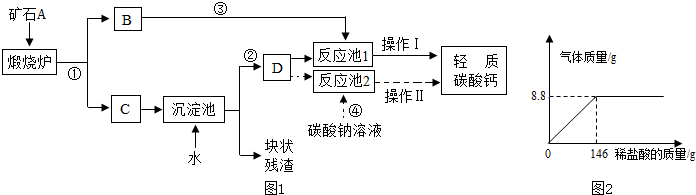
��4����������ʯ��ʯΪԭ�ϣ������Լ���ѡ���������һ���Ʊ�̼��Ƶ�ʵ�鷽����������գ�3����ʵ�鷽��������ͼ��ʾ������
��5�������������Ƿ���̼���ε�ʵ�鷽����
��6��ijѧ��Ϊ�˲ⶨһ����̼���ΪĦ�����������е�̼��ƺ���������ȡ�����������20.0g�����ձ��У������ձ��л�������ϡ����ֱ������������ų��������������������ʶ����������ᷴӦ�������壩����ȥϡ����40.0g����Ӧ��ϺƵ��ձ������ʵ�������Ϊ55.6g���������������ݾ������ձ��������������������������̼��Ƶ���������������ϡ���������������
��1���±��г������������е�Ħ���������ڱ�����д����Ħ�����������������
�� ѡ��ᡱ����������Ρ������������
| �Ϻ���Ч���� | �Ϻ��������� | ���Ӿ����� | |
| Ħ������ | �������� | ̼��� | �������� |
| Ħ������������� | �� �� |
�� �� |
������ ������ |
����
����
������ܡ������ܡ�����3�������е�̼���Ħ����������ʯ��ʯ���Ʊ���ijѧ�������һ���Ʊ�̼��Ƶ�ʵ�鷽����������ͼΪ��
ʯ��ʯ
| 1���� |
| 2��ˮ |
| 3��̼�����Һ |
��д�������������йط�Ӧ�Ļ�ѧ����ʽ����
CaCO3
CaO+CO2��
| ||
CaCO3
CaO+CO2��
����
| ||
CaO+H2O�TCa��OH��2
CaO+H2O�TCa��OH��2
����Ca��OH��2+K2CO3�T2KOH+CaCO3��
Ca��OH��2+K2CO3�T2KOH+CaCO3��
����4����������ʯ��ʯΪԭ�ϣ������Լ���ѡ���������һ���Ʊ�̼��Ƶ�ʵ�鷽����������գ�3����ʵ�鷽��������ͼ��ʾ������
ʯ��ʯ
��ʯ��
ʯ��ˮ
̼���
| ���� |
| ��ˮ |
| ͨ�������̼ |
ʯ��ʯ
��ʯ��
ʯ��ˮ
̼���
������Ƶķ������ŵ���| ���� |
| ��ˮ |
| ͨ�������̼ |
������̼ԭ����
������̼ԭ����
����5�������������Ƿ���̼���ε�ʵ�鷽����
�������м���������ϡ���ᣬ������ɵ�������ʹ�����ʯ��ˮ����ǣ���֤�������к���̼����
�������м���������ϡ���ᣬ������ɵ�������ʹ�����ʯ��ˮ����ǣ���֤�������к���̼����
����6��ijѧ��Ϊ�˲ⶨһ����̼���ΪĦ�����������е�̼��ƺ���������ȡ�����������20.0g�����ձ��У������ձ��л�������ϡ����ֱ������������ų��������������������ʶ����������ᷴӦ�������壩����ȥϡ����40.0g����Ӧ��ϺƵ��ձ������ʵ�������Ϊ55.6g���������������ݾ������ձ��������������������������̼��Ƶ���������������ϡ���������������
��������1���������ʵ�Ԫ����ɺ���������ص㣬�ж����ʵ����
��2��Ħ������Ϊ��Ħ���õģ���������ˮ��
��3�����ݷ�Ӧ��������������д��ѧ����ʽ��
��4����������֮������Ӧ���ʵ����ȡ���ʣ�������Ŀ���ṩ�ķ������жԱȣ���ȷ��������Խ�ԣ�������̼ԭ���ã�
��5������̼���εķ����ǣ��������м���������ϡ���ᣬ������ɵ�������ʹ�����ʯ��ˮ����ǣ���֤�������к���̼���Σ�
��6�����������غ㶨�����������̼���������ݴ����ݷ���ʽ����̼��Ƶ��������������ʵ��������������������̼��Ƶ���������������ϡ���������������
��2��Ħ������Ϊ��Ħ���õģ���������ˮ��
��3�����ݷ�Ӧ��������������д��ѧ����ʽ��
��4����������֮������Ӧ���ʵ����ȡ���ʣ�������Ŀ���ṩ�ķ������жԱȣ���ȷ��������Խ�ԣ�������̼ԭ���ã�
��5������̼���εķ����ǣ��������м���������ϡ���ᣬ������ɵ�������ʹ�����ʯ��ˮ����ǣ���֤�������к���̼���Σ�
��6�����������غ㶨�����������̼���������ݴ����ݷ���ʽ����̼��Ƶ��������������ʵ��������������������̼��Ƶ���������������ϡ���������������
����⣺��1�������������������Ӻ�������������ɵĻ�������ڼ
̼������ɽ������Ӻ����������ɵĻ���������Σ�
����������ֻ�й�Ԫ�غ���Ԫ����ɵĻ�������������
�ʴ�Ϊ����Σ������
��2������Ħ������Ϊ��Ħ���õģ�Ӧ��������ˮ��
�ʴ�Ϊ�����ܣ�
��3��ʯ��ʯ����Ҫ�ɷ�̼��Ƹ��·ֽ����������ƺͶ�����̼����ʯ�Һ�ˮ��Ӧ�����������ƣ��������ƺ�̼��ط�Ӧ����̼��Ƴ������������أ�
�ʴ�Ϊ��
CaCO3
CaO+CO2����
CaO+H2O�TCa��OH��2��
Ca��OH��2+K2CO3�T2KOH+CaCO3����
��4��������ʯ��ʯΪԭ�ϣ����Ƚ�ʯ��ʯ���·ֽ⣬���ɵ���������ˮ��Ӧ�����������ƣ��ٽ����������������̼��Ӧ����̼��ƣ���Ӧ����ͼ�ǣ�
ʯ��ʯ
��ʯ��
ʯ��ˮ
̼��ƣ��÷�����������Ƶķ�����ȣ�������̼���õ���
�ʴ�Ϊ��
ʯ��ʯ
��ʯ��
ʯ��ˮ
̼��ƣ�
������̼ԭ���ã�
��5��̼�����ܺ�ϡ���ᷴӦ���ɶ�����̼�����ô�����������ij�������Ƿ���̼���Σ�
�ʴ�Ϊ���������м���������ϡ���ᣬ������ɵ�������ʹ�����ʯ��ˮ����ǣ���֤�������к���̼���Σ�
��6�����������غ㶨�ɿ�֪����Ӧ���ɵĶ�����̼��������20.0g+40.0g-55.6g=4.4g
��20.0g�����к�CaCO3��������x����Ӧ�к�ȥHCl��������y
CaCO3+2HCl�TCaCl2 +CO2��+H2O
100 73 44
x y 4.4g
=
=
x=10.0g y=7.3g
��������̼��Ƶ���������Ϊ��
��100%=50%��
ϡ���������������
��100%=18.25%
������������̼��Ƶ���������������ϡ��������������ֱ���50%��18.25%��
̼������ɽ������Ӻ����������ɵĻ���������Σ�
����������ֻ�й�Ԫ�غ���Ԫ����ɵĻ�������������
�ʴ�Ϊ����Σ������
��2������Ħ������Ϊ��Ħ���õģ�Ӧ��������ˮ��
�ʴ�Ϊ�����ܣ�
��3��ʯ��ʯ����Ҫ�ɷ�̼��Ƹ��·ֽ����������ƺͶ�����̼����ʯ�Һ�ˮ��Ӧ�����������ƣ��������ƺ�̼��ط�Ӧ����̼��Ƴ������������أ�
�ʴ�Ϊ��
CaCO3
| ||
CaO+H2O�TCa��OH��2��
Ca��OH��2+K2CO3�T2KOH+CaCO3����
��4��������ʯ��ʯΪԭ�ϣ����Ƚ�ʯ��ʯ���·ֽ⣬���ɵ���������ˮ��Ӧ�����������ƣ��ٽ����������������̼��Ӧ����̼��ƣ���Ӧ����ͼ�ǣ�
ʯ��ʯ
| ���� |
| ��ˮ |
| ͨ�������̼ |
�ʴ�Ϊ��
ʯ��ʯ
| ���� |
| ��ˮ |
| ͨ�������̼ |
������̼ԭ���ã�
��5��̼�����ܺ�ϡ���ᷴӦ���ɶ�����̼�����ô�����������ij�������Ƿ���̼���Σ�
�ʴ�Ϊ���������м���������ϡ���ᣬ������ɵ�������ʹ�����ʯ��ˮ����ǣ���֤�������к���̼���Σ�
��6�����������غ㶨�ɿ�֪����Ӧ���ɵĶ�����̼��������20.0g+40.0g-55.6g=4.4g
��20.0g�����к�CaCO3��������x����Ӧ�к�ȥHCl��������y
CaCO3+2HCl�TCaCl2 +CO2��+H2O
100 73 44
x y 4.4g
| 100 |
| x |
| 44 |
| 4.4g |
| 73 |
| y |
| 44 |
| 4.4g |
x=10.0g y=7.3g
��������̼��Ƶ���������Ϊ��
| 10.0g |
| 20.0g |
ϡ���������������
| 7.3g |
| 40.0g |
������������̼��Ƶ���������������ϡ��������������ֱ���50%��18.25%��
������������һ����Ϊ�ۺϵ�ʵ��̽���⣬�ȿ��������ʵ����ʵ�鷽������ƣ��ֿ��������ݷ���ʽ�ļ����֪ʶ���ܺõĿ�����ѧ��Ӧ��֪ʶ��������������

��ϰ��ϵ�д�
 Ӧ������ҵ��ϵ�д�
Ӧ������ҵ��ϵ�д�
�����Ŀ
�������̽��Ҫ�õ����ѧ֪ʶ��
��1���±��г�����������Ħ�����������������
��2����������Ʋ⣬����Ħ�������ܽ����� ������ܡ������ܡ�����
��3�������е�Ħ����֮һ̼��ƿ�����ʯ��ʯ���Ʊ���ijѧ�������һ���Ʊ�̼��Ƶ�ʵ�鷽����������ͼΪ��

��д�������������йط�Ӧ�Ļ�ѧ����ʽ��
�� ���� ���� ��
��4����������ʯ��ʯΪԭ�ϣ������Լ���ѡ���������һ���Ʊ�̼��Ƶ�ʵ�鷽�������գ�3����ʾ�������ʵ�鷽��������ͼ��ʾ������
����Ƶķ����ŵ��� ��
��5�������������Ƿ���̼���ε�ʵ�鷽���� ��
��1���±��г�����������Ħ�����������������
| ���������� | �������������� | ������� | |
| Ħ���� | �������� | ̼��� | �������� |
| Ħ�������������ָ�ᡢ��Ρ������ |
��3�������е�Ħ����֮һ̼��ƿ�����ʯ��ʯ���Ʊ���ijѧ�������һ���Ʊ�̼��Ƶ�ʵ�鷽����������ͼΪ��

��д�������������йط�Ӧ�Ļ�ѧ����ʽ��
��
��4����������ʯ��ʯΪԭ�ϣ������Լ���ѡ���������һ���Ʊ�̼��Ƶ�ʵ�鷽�������գ�3����ʾ�������ʵ�鷽��������ͼ��ʾ������
����Ƶķ����ŵ���
��5�������������Ƿ���̼���ε�ʵ�鷽����
̽�������⣺
�������̽��Ҫ�õ����ѧ֪ʶ��
��1���±��г������������е�Ħ���������ڱ�����д����Ħ�����������������
��2����������Ʋ⣬����Ħ�������ܽ����� ������ܡ������ܡ�����
��3�������е�Ħ����̼��ƿ�����ʯ��ʯ���Ʊ�����ѧ�������һ���Ʊ�̼��Ƶ�ʵ�鷽����������ͼΪ��

��д�������������йػ�ѧ�Ļ�ѧ����ʽ��
�� ���� ���� ��
��4����������ʯ��ʯΪԭ�ϣ������Լ���ѡ���������һ���Ʊ�̼��Ƶ�ʵ�鷽�������գ�3����ʾ�������ʵ�鷽��������ͼ��ʾ������
ʯ��ʯ
����Ƶķ����ŵ��� ��
��5�������������Ƿ���̼���ε�ʵ�鷽���� ��
��6��ijѧ��Ϊ�˲ⶨһ����̼���ΪĦ������������̼��Ƶĺ��������ձ���ȡ�����������100.0g�����ձ�������ϡ����������������ų�����̼����⣬���������е��������ʲ��������ᷴӦ�������壩������ȥϡ����200.0g����Ӧ��Ϻ�Ƶ��ձ������ʵ�����Ϊ278.0g���������������ݾ������ձ��������������������������̼��Ƶ�����������
�������̽��Ҫ�õ����ѧ֪ʶ��
��1���±��г������������е�Ħ���������ڱ�����д����Ħ�����������������
| ��ͯ���� | �������������� | ������������ | |
| Ħ���� | �������� | ̼��� | �������� |
| ���ʵ���� ��ָ�ᡢ��Σ� |
��3�������е�Ħ����̼��ƿ�����ʯ��ʯ���Ʊ�����ѧ�������һ���Ʊ�̼��Ƶ�ʵ�鷽����������ͼΪ��

��д�������������йػ�ѧ�Ļ�ѧ����ʽ��
��
��4����������ʯ��ʯΪԭ�ϣ������Լ���ѡ���������һ���Ʊ�̼��Ƶ�ʵ�鷽�������գ�3����ʾ�������ʵ�鷽��������ͼ��ʾ������
ʯ��ʯ
����Ƶķ����ŵ���
��5�������������Ƿ���̼���ε�ʵ�鷽����
��6��ijѧ��Ϊ�˲ⶨһ����̼���ΪĦ������������̼��Ƶĺ��������ձ���ȡ�����������100.0g�����ձ�������ϡ����������������ų�����̼����⣬���������е��������ʲ��������ᷴӦ�������壩������ȥϡ����200.0g����Ӧ��Ϻ�Ƶ��ձ������ʵ�����Ϊ278.0g���������������ݾ������ձ��������������������������̼��Ƶ�����������



 ��ش��������⣺
��ش��������⣺