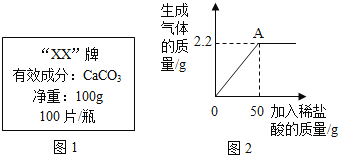
��Ŀ����
����Ŀ���ϳ����ǹ�ҵ�����е�һ��ԭ��������Ҫ�ɷ���һ����̼��������������ұ�����������������ѵȡ������ͼʾ�ش�

��ע��ͼ�������ڻ�ѧʽ��ʾ��Ӧ���ʵ���Ҫ�ɷ֣�
��1����д�����úϳ��������Ļ�ѧ����ʽ_____��дһ�����ɣ���
��2�������ѣ�CH3OCH3������Ϊ21��������ȼ�ϣ���ʵ�ָ�Ч���ȼ�գ���д���������ڿ����г��ȼ�����ɶ�����̼��ˮ�Ļ�ѧ����ʽ_____��
��3���ϳ����ڲ�ͬ���������£����Ժϳɲ�ͬ�����ʡ����úϳ���Ϊԭ�ϲ����ܵõ���������_____������ĸ��ţ�������_____��
A �״���CH3OH�� B �Ҷ�ȩ��HC2O2�� C ����[CO��NH2��2]
���𰸡�Fe2O3��3H2![]() 2Fe��3H2O��Fe2O3��3CO
2Fe��3H2O��Fe2O3��3CO![]() 2Fe��3CO2 CH3OCH3��3O2
2Fe��3CO2 CH3OCH3��3O2![]() 2CO2��3H2O C �����к��е�Ԫ�أ��ϳ�����ֻ����̼���⡢������Ԫ�أ�����Ԫ���غ�
2CO2��3H2O C �����к��е�Ԫ�أ��ϳ�����ֻ����̼���⡢������Ԫ�أ�����Ԫ���غ�
��������
��1��һ����̼���������ڸ��������·�Ӧ�������Ͷ�����̼�Ļ�ѧ����ʽ��Fe2O3��3CO![]() 2Fe��3CO2���������������ڼ��������·�Ӧ��������ˮ�Ļ�ѧ����ʽ��Fe2O3��3H2
2Fe��3CO2���������������ڼ��������·�Ӧ��������ˮ�Ļ�ѧ����ʽ��Fe2O3��3H2![]() 2Fe��3H2O��
2Fe��3H2O��
��2���������ڿ����г��ȼ�����ɶ�����̼��ˮ�Ļ�ѧ����ʽ��CH3OCH3��3O2![]() 2CO2��3H2O��
2CO2��3H2O��
��3���ϳ����ڲ�ͬ���������£����Ժϳɲ�ͬ�����ʡ����úϳ���Ϊԭ�ϲ����ܵõ��������ǣ����أ������ǣ������к��е�Ԫ�أ��ϳ�����ֻ����̼���⡢������Ԫ�أ�����Ԫ���غ㡣
��ѡ��C��

 �γ̴����Ծ�����100��ϵ�д�
�γ̴����Ծ�����100��ϵ�д� �¾�����ĩ���100��ϵ�д�
�¾�����ĩ���100��ϵ�д� ȫ�ܴ���100��ϵ�д�
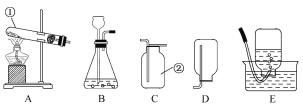
ȫ�ܴ���100��ϵ�д�����Ŀ��ij��ɫ������ܺ��� BaC12����Һ�����ԣ���NaOH��Na2CO3��Na2SO4 �е�һ�ֻ��֣�ijС��ͬѧΪȷ���ð�ɫ����ijɷ֣���������̽����
�� ����̽����ʵ���������ͼ��ʾ��
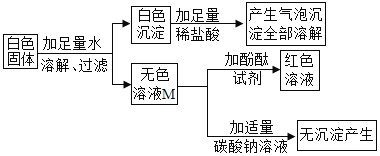
���������٣�ԭ��ɫ������һ���е�������_____________��һ��û�е�������_____________��M��Һ�е�����һ����_____________��һ��û�� BaCl2 ��������_____________���û�ѧ����ʽ��ʾ��
���������ۣ���ͬѧ��Ϊ����ɫ��Һ M ���ɫ��˵����Һ M �к��м������ʣ�������˵��ԭ��ɫ�������Ƿ����������ƣ���Ҫ��һ��ȷ����
�� ����̽������ɫ��Һ M ���Ƿ����������ƣ�
��ʵ��̽����
ʵ �� �� �� | ʵ �� �� �� | ʵ �� Ŀ �� | ʵ����� |
Aȡ��Һ M ���Թ��У�����______�������á� | ��ɫ���� | _______ | ______ |
Bȡʵ�� A ���õ��ϲ���Һ���Թ��У�����____�Լ��� | _____ | ֤����Һ M ���Ƿ����������� | ԭ��ɫ�����к����������� |
ͨ��ʵ�飬С��ͬѧ����˱���̽�����
����Ŀ��������û����������ʱ������ˮ��������Ӧ���������£�����ˮ�����ܷ�Ӧ����һ�ֳ����������������һ�����塣С���ܺ��棬�����ͼ��ʾʵ�飬̽��������ˮ������Ӧ��IJ��

��1���Թ�β����һ��ʪ����Ŀ����_____��
���������ϣ�
��2��̽�����ɵ�������ʲô��
��ȼ�ŵ�ľ�����������ݣ��б��������Ժ��з�����Ʈ�����С�˵�����ɵ�������_____��
��3��̽���Թ���ʣ�����ɷ���ʲô��
�������������� | FeO | Fe2O3 | Fe3O4 |
��ɫ��״̬ | ��ɫ��ĩ | ����ɫ��ĩ | ��ɫ���� |
�ܷ������� | �� | �� | �� |
��������֤���Թ���ʣ�����Ϊ��ɫ����ȫ���������������ú�ɫ���岻������_____������ˮ������Ӧ�Ļ�ѧ����ʽΪ_____��
����������裩����һ��ʣ�������Fe��Fe3O4���������ʣ�������_____��
���������ϣ�![]() ��
��
��ʵ��̽����С����������ʵ��̽����ȡ��ʣ������������Թ��У�����������ϡ���ᣬ�۲쵽_____���ó����ۡ�����һ��������