��Ŀ����
19���ҹ��нϳ��ĺ����ߣ�������Դʮ�ַḻ�������ǶԺ�ˮ��Դ�IJ������ã�
��1����ˮ�к�����ߵ�����
��ҵ�ϲ������µ����̴Ӻ�ˮ����ȡ���Σ�

���������Т�ֱ���
��2�������к�����ɳ������þ���Ȼ��Ƶ����ʣ�����������ˮ��Ȼ���ٽ������²������ɵõ��ϴ����Ȼ��ƣ��ٹ��ˣ��ڼӹ�����NaOH��Һ���ۼ�����������ܼӹ�����Na2CO3��Һ���ݼӹ�����BaCl2��Һ����ȷ�IJ���˳����
��3���ӡ�ɹ�Ρ����ĸҺ��±ˮ������ȡ��Ҫ����þ����ȡþ�IJ������£�
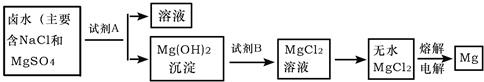
��ȡMg�Ĺ����У��Լ�A���ѡ��
��1����ˮ�к�����ߵ�����
�Ȼ���
����ˮ������
�ᾧ�ɻ�ô��Σ���ҵ�ϲ������µ����̴Ӻ�ˮ����ȡ���Σ�

���������Т�ֱ���
������
���ᾧ��
���ᴿ�����ܽ⡢���˺�����Ҫȫд��
����2�������к�����ɳ������þ���Ȼ��Ƶ����ʣ�����������ˮ��Ȼ���ٽ������²������ɵõ��ϴ����Ȼ��ƣ��ٹ��ˣ��ڼӹ�����NaOH��Һ���ۼ�����������ܼӹ�����Na2CO3��Һ���ݼӹ�����BaCl2��Һ����ȷ�IJ���˳����
�ڢݢܢ٢ۣ���ݢܢڢ٢ۻ�ݢڢܢ٢ۣ�
����3���ӡ�ɹ�Ρ����ĸҺ��±ˮ������ȡ��Ҫ����þ����ȡþ�IJ������£�

��ȡMg�Ĺ����У��Լ�A���ѡ��
��ʯ��
���Լ�Bѡ��ϡ����
�������ˮMgCl2�Ĺ���������
��ת��Ϊ��ѧ��
�ܣ���������1�����ݺ�ˮ�ijɷּ��Ȼ��Ƶ��ܽ�����¶ȵ�Ӱ��������ɣ�
��2�����ݴӺ�ˮ���ᴿʳ�εIJ��������������ɣ�
��3�����ݴӿ�±����ȡ����þ��ʵ�����������
��2�����ݴӺ�ˮ���ᴿʳ�εIJ��������������ɣ�
��3�����ݴӿ�±����ȡ����þ��ʵ�����������
����⣺��1����ˮ�к�������������ˮ��������Ȼ��ƣ�
��Ϊ�Ȼ��Ƶ��ܽ�����¶�Ӱ�첻�����Բ��������ᾧ�ķ�����ȡʳ�Σ�
��ҵ����ȡʳ�εIJ���Ϊ����ˮ������ˮ�أ������˽��������ء��ᾧ�أ������˿ɵõ�ĸҺ�ʹ��Σ����ν�һ���ᴿΪ���Σ�
�ʴ�Ϊ���Ȼ��ơ������ᾧ�������ء��ᾧ�ء��ᴿ��
��2����������ˮ��õ�����Һ�к���MgSO4��CaCl2��NaCl����������Һ�м���������NaOH��Һ����ʱ�õ��Ļ����Ϊ��Mg��OH��2������Na2SO4��Ca��OH��2������NaOH��NaCl������յõ��Ļ���м��������BaCl2��Һ����ʱ�õ��Ļ����Ϊ��Mg��OH��2������BaSO4������Ca��OH��2������NaOH��NaCl��BaCl2�����������м��������
Na2CO3��Һ���õ��Ļ����Ϊ��Mg��OH��2������BaSO4������CaCO3������NaOH��BaCO3������NaCl��
Na2CO3���˻���ᆳ���˺�õ�NaOH��NaCl�Ļ����Һ����ʱ�������������ᣬ���ɵõ��ϴ������Ȼ�����Һ�������ȡ�Ȼ��Ƽ��ɣ�
�ʴ�Ϊ���ڢݢܢ٢�
��3����Ϊ�������зḻ��ʯ��ʯ��Դ������ʯ��ʯ���Եõ������ƣ�������������ˮ���Ƶ�����������Һ������A�Լ�Ӧ������������Һ����ʵ���������£���NaCl��MgSO4�Ļ����Һ�м����Լ�A��Ca��OH��2��ɵõ�Mg��OH��2��������Mg��OH��2�����м���ϡ����ɵõ�MgCl2��Һ��Ȼ�����������ɵõ�Mg��
��ѡCa��OH��2��ϡ���ᡢ���ܡ���ѧ��
��Ϊ�Ȼ��Ƶ��ܽ�����¶�Ӱ�첻�����Բ��������ᾧ�ķ�����ȡʳ�Σ�
��ҵ����ȡʳ�εIJ���Ϊ����ˮ������ˮ�أ������˽��������ء��ᾧ�أ������˿ɵõ�ĸҺ�ʹ��Σ����ν�һ���ᴿΪ���Σ�
�ʴ�Ϊ���Ȼ��ơ������ᾧ�������ء��ᾧ�ء��ᴿ��
��2����������ˮ��õ�����Һ�к���MgSO4��CaCl2��NaCl����������Һ�м���������NaOH��Һ����ʱ�õ��Ļ����Ϊ��Mg��OH��2������Na2SO4��Ca��OH��2������NaOH��NaCl������յõ��Ļ���м��������BaCl2��Һ����ʱ�õ��Ļ����Ϊ��Mg��OH��2������BaSO4������Ca��OH��2������NaOH��NaCl��BaCl2�����������м��������
Na2CO3��Һ���õ��Ļ����Ϊ��Mg��OH��2������BaSO4������CaCO3������NaOH��BaCO3������NaCl��
Na2CO3���˻���ᆳ���˺�õ�NaOH��NaCl�Ļ����Һ����ʱ�������������ᣬ���ɵõ��ϴ������Ȼ�����Һ�������ȡ�Ȼ��Ƽ��ɣ�
�ʴ�Ϊ���ڢݢܢ٢�
��3����Ϊ�������зḻ��ʯ��ʯ��Դ������ʯ��ʯ���Եõ������ƣ�������������ˮ���Ƶ�����������Һ������A�Լ�Ӧ������������Һ����ʵ���������£���NaCl��MgSO4�Ļ����Һ�м����Լ�A��Ca��OH��2��ɵõ�Mg��OH��2��������Mg��OH��2�����м���ϡ����ɵõ�MgCl2��Һ��Ȼ�����������ɵõ�Mg��
��ѡCa��OH��2��ϡ���ᡢ���ܡ���ѧ��
�������������ͼ��ʱҪע�����������Լ��Ƿ������������Ҫ�����Ƿ���Ҫ��ȥ��

��ϰ��ϵ�д�
 �����������Ů��ͯ������ϵ�д�
�����������Ů��ͯ������ϵ�д�
�����Ŀ

 �ҹ��нϳ��ĺ����ߣ�������Դʮ�ַḻ��
�ҹ��нϳ��ĺ����ߣ�������Դʮ�ַḻ��
