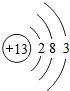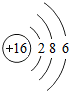��Ŀ����
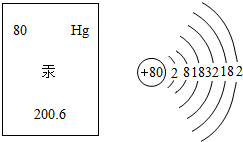
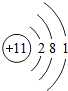
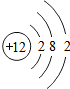
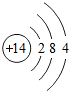
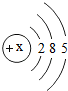
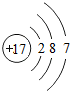
�˵����Ϊ1��18��Ԫ�ص�ԭ�ӽṹʾ��ͼ����Ϣ���£��ش��������⣺
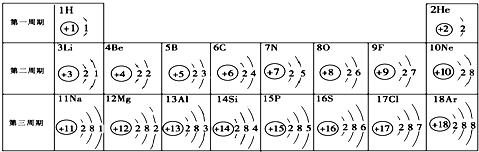
��1���ڵ��������У���ԭ�ӽṹ�Ĺ�֮ͬ����______���������У���ԭ�Ӻ�������Ų��ı仯������______��
��2���ڵ��������У�Ԫ�����͵ı仯����ǣ���������______Ԫ�ع��ɵ�______Ԫ�أ�����ϡ������Ԫ�ؽ�β��
��3��д����������Ų�����ԭ����ͬ�������Ӻ������ӷ��Ÿ�һ����������______��������______��
��4��д�����е�Ԫ�صij������ʡ�������Ļ�ѧʽ��һ����
��5����Ԫ�����ڱ��У�ͬһ�壨���У���Ԫ�ؾ������ƵĻ�ѧ���ʣ������и���Ԫ�ؾ������ƻ�ѧ���ʵ���______������ţ���
A��C��NeB��Be��MgC��Al��SiD��F��Cl
��6����11��Ԫ�����17��Ԫ����ɵĻ�������______��д��ѧʽ�������ɸ����ʵ�����______��ѡ����ӡ�����ԭ�ӡ������ӡ�����

��1���ڵ��������У���ԭ�ӽṹ�Ĺ�֮ͬ����______���������У���ԭ�Ӻ�������Ų��ı仯������______��
��2���ڵ��������У�Ԫ�����͵ı仯����ǣ���������______Ԫ�ع��ɵ�______Ԫ�أ�����ϡ������Ԫ�ؽ�β��
��3��д����������Ų�����ԭ����ͬ�������Ӻ������ӷ��Ÿ�һ����������______��������______��
��4��д�����е�Ԫ�صij������ʡ�������Ļ�ѧʽ��һ����
| ���ʵ���� | ���� | ������ |
| ���ʵĻ�ѧʽ |
A��C��NeB��Be��MgC��Al��SiD��F��Cl
��6����11��Ԫ�����17��Ԫ����ɵĻ�������______��д��ѧʽ�������ɸ����ʵ�����______��ѡ����ӡ�����ԭ�ӡ������ӡ�����
��1���ڵ��������У���ԭ�ӽṹ�Ĺ�֮ͬ����ԭ�Ӻ�����Ӳ�����ͬ���������У������ң���ԭ�Ӻ�������Ų��ı仯�����������������������ӣ����ԭ�Ӻ�����Ӳ�����ͬ�������������������ӣ�
��2���ڵ��������У�Ԫ�����͵ı仯����ǣ��������ɽ���Ԫ�ع��ɵ��ǽ���Ԫ�أ�����ϡ������Ԫ�ؽ���������������ǽ�����
��3����������Ų�������ԭ����ͬ�������ӿ����������ӻ�þ�����Լ������ӣ������ӿ����������ӡ������ӣ����Na+��F-��
��4�����е�Ԫ�صĵ����ǵ������������Ԫ�صĻ��ϼ���+1��+2��+3��+4��+5�ۣ�����Ϊ-2�ۣ����Զ�Ӧ��������N2O��NO��N203��NO2��N2O5�ȣ�
�ʴ𰸣�N2��NO2��
��5���������⣬ͬһ�壨���У���Ԫ�ؾ������ƵĻ�ѧ���ʣ�Be��Mg��F��Cl����ͬһ�壨���У�����ѧ�������ƣ�
�ʴ𰸣�BD��
��6��11����Ԫ������������Ϊ1���ʻ��ϼ�+1�ۣ�17��Ԫ��Ϊ�ȣ��ʻ��ϼ�Ϊ-1�ۣ�����Ԫ�غ���Ԫ����ɵĻ�����Ļ�ѧʽΪNaCl�����ɸ����ʵ����������Ӻ������ӣ��ʴ𰸣�NaCl�����ӣ�
��2���ڵ��������У�Ԫ�����͵ı仯����ǣ��������ɽ���Ԫ�ع��ɵ��ǽ���Ԫ�أ�����ϡ������Ԫ�ؽ���������������ǽ�����
��3����������Ų�������ԭ����ͬ�������ӿ����������ӻ�þ�����Լ������ӣ������ӿ����������ӡ������ӣ����Na+��F-��
��4�����е�Ԫ�صĵ����ǵ������������Ԫ�صĻ��ϼ���+1��+2��+3��+4��+5�ۣ�����Ϊ-2�ۣ����Զ�Ӧ��������N2O��NO��N203��NO2��N2O5�ȣ�
�ʴ𰸣�N2��NO2��
��5���������⣬ͬһ�壨���У���Ԫ�ؾ������ƵĻ�ѧ���ʣ�Be��Mg��F��Cl����ͬһ�壨���У�����ѧ�������ƣ�
�ʴ𰸣�BD��
��6��11����Ԫ������������Ϊ1���ʻ��ϼ�+1�ۣ�17��Ԫ��Ϊ�ȣ��ʻ��ϼ�Ϊ-1�ۣ�����Ԫ�غ���Ԫ����ɵĻ�����Ļ�ѧʽΪNaCl�����ɸ����ʵ����������Ӻ������ӣ��ʴ𰸣�NaCl�����ӣ�

��ϰ��ϵ�д�
 ʱ�����������ҵԭ���ܳ�����ϵ�д�
ʱ�����������ҵԭ���ܳ�����ϵ�д� ����νӽ̲���ĩ���Ԥϰ�人������ϵ�д�
����νӽ̲���ĩ���Ԥϰ�人������ϵ�д� ������ҵ��ٳɳ����½������������ϵ�д�
������ҵ��ٳɳ����½������������ϵ�д�
�����Ŀ