��Ŀ����
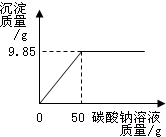
С��ͬѧ��ij�������������ʵ��������Ա��С��һ��������Ȼ������Ȼ�����ɵIJ�Ʒ���Ȼ��Ƶ�����������ȡ16.25g������Ʒ��ȫ������143.6mLˮ�У������õ��Ļ����Һ����μ���������������Ϊ10.6%��̼������Һ����¼����ͼ��ʾ�����߹�ϵ��
����Ա��С�����ʾ��
�ٷ�Ӧ�Ļ�ѧ����ʽBaCl2+Na2CO3==BaCO3��+2NaCl
��ˮ���ܶ�Ϊ1g/cm3
����Է�������BaCl2��208 Na2CO3��106 BaCO3��197 NaCl��58.5
����Ա��С�����ʾ��
�ٷ�Ӧ�Ļ�ѧ����ʽBaCl2+Na2CO3==BaCO3��+2NaCl
��ˮ���ܶ�Ϊ1g/cm3
����Է�������BaCl2��208 Na2CO3��106 BaCO3��197 NaCl��58.5

�ŵ��Ȼ�����̼����ǡ����ȫ��Ӧʱ������10.6%��̼������Һ��������________g��
�Ʋ�Ʒ���Ȼ��Ƶ����������Ƕ��٣�
�ǵ��Ȼ�����̼����ǡ����ȫ��Ӧʱ�����ˣ�������Һ�����ʵ����������Ƕ��٣�
�Ʋ�Ʒ���Ȼ��Ƶ����������Ƕ��٣�
�ǵ��Ȼ�����̼����ǡ����ȫ��Ӧʱ�����ˣ�������Һ�����ʵ����������Ƕ��٣�
��50
�ƽ⣺����Ʒ��BaCl2������Ϊx������NaCl������Ϊy��
����Na2CO3+BaCl2==BaCO3��+2NaCl
����106������208������������117
50g��10.6������x��������������y
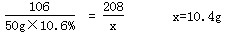
��Ʒ��NaCl����������Ϊ
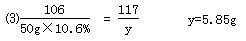
��Һ�����ʵ���������Ϊ
�ƽ⣺����Ʒ��BaCl2������Ϊx������NaCl������Ϊy��
����Na2CO3+BaCl2==BaCO3��+2NaCl
����106������208������������117
50g��10.6������x��������������y
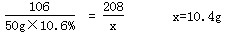
��Ʒ��NaCl����������Ϊ

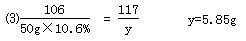
��Һ�����ʵ���������Ϊ


��ϰ��ϵ�д�
�����Ŀ
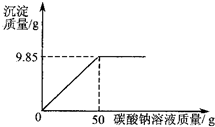 С��ͬѧ��ij�������������ʵ��������Ա��С��һ��������Ȼ������Ȼ�����ɵIJ�Ʒ���Ȼ��Ƶ�����������ȡ16.25g������Ʒ��ȫ������143.6mLˮ�У������õ��Ļ����Һ����μ���������������Ϊ10.6%��̼������Һ����¼����ͼ��ʾ�����߹�ϵ��
С��ͬѧ��ij�������������ʵ��������Ա��С��һ��������Ȼ������Ȼ�����ɵIJ�Ʒ���Ȼ��Ƶ�����������ȡ16.25g������Ʒ��ȫ������143.6mLˮ�У������õ��Ļ����Һ����μ���������������Ϊ10.6%��̼������Һ����¼����ͼ��ʾ�����߹�ϵ��