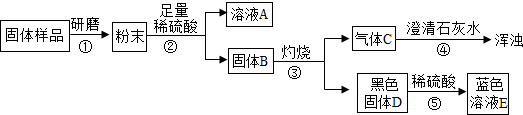
��Ŀ����
��2010?���죩��ͼ�е����ʾ�Ϊ���л�ѧ�α����������ʣ��������ڵ��ʵ���B��F��H��L���������������C��D��E��G��J��C�dz��ʯ�е���Ҫ�ɷ֣�DΪ��ɫ��ζ��������ˮ���γ����ꣻG���������ЧӦ��J�������к����������ʣ�
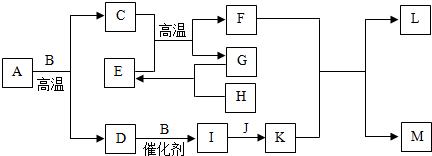
��1��B��������
��2��G��H��Ӧʱ
��3��д��C��E��Ӧ�Ļ�ѧ����ʽ��
��4��Ϊ�˼���D���ŷţ��ɲ�ȡ�Ĵ�ʩ�У�

��1��B��������
����
����
��ѡ����ӡ�����ԭ�ӡ������ӡ������ɵģ�M�������ӵķ���ΪFe2+
Fe2+
����2��G��H��Ӧʱ
����
����
��ѡ��ų��������ա�����������3��д��C��E��Ӧ�Ļ�ѧ����ʽ��
3CO+Fe2O3
2Fe+3CO2
| ||
3CO+Fe2O3
2Fe+3CO2
��
| ||
��4��Ϊ�˼���D���ŷţ��ɲ�ȡ�Ĵ�ʩ�У�
����ú��ʹ�ã�ʹ������ú����������Դ��
����ú��ʹ�ã�ʹ������ú����������Դ��
��дһ���������������ȣ��ҵ�����ͻ�ƿڣ�����ؼ���Ȼ��˳�����ϡ�����˳�ơ����ƻ�˳���������ϣ��ó����ۣ������ԭ����飮C�dz�������Ҫ�ɷ֣�˵��C����������D���γ���������壬˵��D�Ƕ�������G���������ЧӦ��˵��G�Ƕ�����̼��J�������к����������ʣ�˵��J��ˮ��
����⣺��������ϢC�dz�������Ҫ�ɷ֣�˵��C����������D���γ���������壬˵��D�Ƕ�������A��B��Ӧ�����������Ͷ�������A��FeS2��B������������������������Ӧ������I����������J�������к����������ʣ�˵��J��ˮ��������������ˮ�γ�K���ᣬ��������E��Ӧ����G��G���������ЧӦ��˵��G�Ƕ�����̼����E��һ����̼�� һ����̼����������Ӧ�������Ͷ�����̼���������ᷴӦ��������������������
�ʴ�Ϊ��
��1�����ӣ� Fe2+��
��2�����գ�
��3����3CO+Fe2O3
2Fe+3CO2��
��4��������ú��ʹ�ã�ʹ������ú����������Դ��
�ʴ�Ϊ��
��1�����ӣ� Fe2+��
��2�����գ�
��3����3CO+Fe2O3
| ||
��4��������ú��ʹ�ã�ʹ������ú����������Դ��
����������ؼ����������ͻ�ƿڣ�����ͻ�ƿڳ�����̽��֪ʶ���������ϵ��Ӧ�ö���˼ά��ʽ���������ܵķ��������������Ƴ���������Ľ����

��ϰ��ϵ�д�
�����Ŀ
 ��2010?���죩���������ܺ���չܵ���Ҫ���ϣ��Ԫ�أ�Ԫ�ط���ΪRb��ԭ�ӽṹʾ��ͼ����ͼ��ʾ�������й��Ԫ�ص�˵���У�����ȷ���ǣ�������
��2010?���죩���������ܺ���չܵ���Ҫ���ϣ��Ԫ�أ�Ԫ�ط���ΪRb��ԭ�ӽṹʾ��ͼ����ͼ��ʾ�������й��Ԫ�ص�˵���У�����ȷ���ǣ�������