��Ŀ����
ij��ѧ��ȤС�飬��һ�β��ġ����������������θ����ࡱ�����Ϻ�֪��0.84%С�մ�ˮ��NaHCO3ˮ��Һ�������ڻ���θ�����֢״�����Ǿ�������ʵ���������������������������С�մ�ˮ��NaHCO3ˮ��Һ��1000�ˣ�
��1�����������������ù��̣�
��һ����ȷ����______��ʳƷ��С�մ��ĩ��
�ڶ�������������С�մ��ĩ���뾭�������ձ��У����ձ��м�������ˮ______�ˣ�����ܽ⣬���õ�����С�մ�ˮ��
��2��θ���������ҽ����ָ���£�ÿ�κ�����С�մ�ˮ50�ˣ�ÿ��2�Σ���һ��ɷ�Ӧ��θҺ�е�HCl���ٿˣ�
�⣺��1��С�մ�����=1000g��0.84%=8.4g��ˮ��������1000g-8.4g=991.6g��
��2��ÿ�������С�մ������50g��2��0.84%=0.84g��
��ɷ�Ӧ��θҺ�е�HCl ������ΪX��
NaHCO3+HCl=NaCl+H2O+CO2��
84 36.5
0.84g X
���ݣ�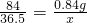 ���X=0.365g
���X=0.365g
�ʴ�Ϊ����1��8.4��991.6����2��0.365g��
��������1��������������=��Һ���������������������㣻�����ܼ�������=��Һ������-�����������㣻��2������С�մ���ϡ���ᷴӦ�Ļ�ѧ����ʽ���
������������С�մ�����θ�����Ϊ�������ʵ��̽������Һ�������õ����������ʵ������������㣬���ݷ���ʽ�ļ��㣬�����ӱ��������ѧ����Ӧ����������ᵽѧϰ��ѧ��ʵ������
��2��ÿ�������С�մ������50g��2��0.84%=0.84g��
��ɷ�Ӧ��θҺ�е�HCl ������ΪX��
NaHCO3+HCl=NaCl+H2O+CO2��
84 36.5
0.84g X
���ݣ�
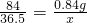 ���X=0.365g
���X=0.365g�ʴ�Ϊ����1��8.4��991.6����2��0.365g��
��������1��������������=��Һ���������������������㣻�����ܼ�������=��Һ������-�����������㣻��2������С�մ���ϡ���ᷴӦ�Ļ�ѧ����ʽ���
������������С�մ�����θ�����Ϊ�������ʵ��̽������Һ�������õ����������ʵ������������㣬���ݷ���ʽ�ļ��㣬�����ӱ��������ѧ����Ӧ����������ᵽѧϰ��ѧ��ʵ������

��ϰ��ϵ�д�
�����Ŀ