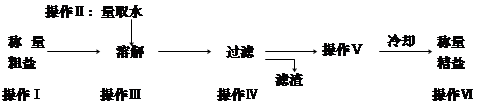��Ŀ����
ij̼������Ʒ�к��������Ȼ������ʣ�Ϊ�ⶨ����Ʒ��̼���Ƶ���������������������ʵ�飺
�� ʵ��һ��
��ش��������⣺
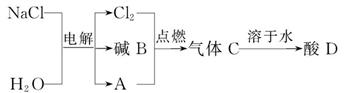
������A���õ����������������� ��
����ͬѧȡ������̼������Ʒ����Һ��������ɫ��̪��Һ����Һ����ɫ����� ��
������ʵ������м��뱥��ʯ��ˮ������Ӧ�Ļ�ѧ����ʽ�� ��
������Ϊ̽��������Ӧ����Һ�е����ʳɷ֣���ͬѧ����Һ�еμӹ���ϡ���ᣬ����
�����ݲ�������μ�����ǰ��Һ�е����ʳ��Ȼ������ ��
�� ʵ�����
��ͬѧȡ12g��̼������Ʒ�����ձ��У�����100gϡ���ᣨ����������ȫ��Ӧ�� ��
����Һ����Ϊ107.6 g���Լ��㣺
�������ɶ�����̼���ʵ���Ϊ mol��
����̼������Ʒ��Na2CO3��������������д��������̣�������0.1%��
���������õĽ����ʵ�ʵ����������ߣ����ܵ�ԭ���� ������һ�ּ��ɣ�
��ʵ��һ
�������� ����ɫ ����Na2CO3 +Ca(OH)2 = 2NaOH +CaCO3��
������̼���ơ��������ƣ�д��ѧʽҲ�ɣ�������©һ�����÷֣�
��ʵ���������0.1mol
����88.3%
����ϡ����ӷ�����HCl������CO2�����ų����Ӷ�����������Ʒ��̼���Ƶ���������ƫ����ˮ������CO2�����ų����Ӷ�����������Ʒ��̼���Ƶ���������ƫ��1�֣�
�������������̼������Ʒ�к��������Ȼ������ʣ���ˮ�ܽ��õ�̼������Һ���������Ȼ��ƣ���Һ�ʼ��ԣ��ӷ�̪Ӧ���ɫ��̼�����ܺ��������Ʒ�Ӧ����ѧ����ʽΪNa2CO3 +Ca(OH)2 = 2NaOH +CaCO3�����г������ɣ�����A����Ϊ���ˣ�����������Ϊ�������μӹ���ϡ���ᣬ���������ݲ�������һ����̼���ƣ�������������Ҳһ���У���Ϊ������Ӧ�������ɳ�����Ҳ�������������ƣ�������Һ�л����������ƺ�̼���ƣ����������٣����ٵ�������Ϊ���ɶ�����̼��������Ϊ112-107.6=4.4g�����ʵ���Ϊ0.1mol��
Ȼ����ݻ�ѧ����ʽ���㣺����Ʒ�к�̼����Xmol
Na2CO3��2HCl=2NaCl��H2O��CO2��
1mol 1mol
x 0.1g
1��1= x��0.1 x = 0.1mol
��Ʒ��̼���Ƶ���������Ϊ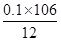 ��100% = 88.3%
��100% = 88.3%
�����õĽ����ʵ�ʵ����������ߣ��������̼����Ӧ��ƫ���ɴ˿��Ƴ�ԭ�����Ϊϡ����ӷ�����HCl������CO2�����ų���ˮ������CO2�����ų������²ⶨ�Ķ�����̼��ƫ�����������̼���Ƶ���������ƫ��
���㣺ʵ��̽�������ݻ�ѧ����ʽ���㣬��Һ�����ʵ��жϣ����ķ����ȡ�

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�ά����C(���Vc����������Ѫ��)��������ˮ���ױ�����������ȱ��Vc�����������ּ�����ˮ�����߲��к��зḻ��Vc��ij�о���ѧϰС�����̽�����£�
̽��һ���ⶨ������Vc�ĺ�����
���������ϡ�Vc�ܺ�����ط�Ӧ��ʹ��ɫ�ĸ��������Һ��ɫ��
����Ʒ������ֱ���ʢ��1 mL��Ũ�ȸ������ϡ��Һ����֧�Թ�����εμӹ�ζ���ϡ�ƻ��֭����֭��0.04%��Vc��Һ���ߵα���ֱ�����������Һ��ɫ��
��ʵ�����ݡ�
| | ��ζ���� | ƻ��֭ | ��֭ | 0.04%��Vc��Һ |
| �μӵĵ��� | 40 | 10 | 20 | 5 |
��ʵ����ۡ��������ݿ�֪��Vc������ߵ��� ������Ϊ (��Һ���ܶ��ϵIJ���ÿһ�ε���������Բ���)��
̽�������߲˷���ʱ��ij��̶���Vc�����Ƿ���Ӱ�졣
����Ʒ��������������ʵĻƹϡ�����һ�ܵĻƹϡ��������ϡ��Һ�ͱ�Ҫ���������ʵ�鷽���� ��
��ʵ����ۡ�����������ʵ�飬���� ��һʵ�����������ó��߲˷���ʱ��ij��̶���Vc�ĺ�����Ӱ�졣
��ʵ�鷴˼����ѧʵ����Ҫ���Ʊ����������������Ӱ�쵽�ⶨ������� ��
A.ÿ��ʵ�����õĹ�֭��ɫ��ͬ
B.��ȡ�ı������ʵ������ͬ
C.�ԹܵĴ�С��ͬ
D.û����ͬһ���ͷ�ιܵμ�
С�����˸������ͣ�����һ�����ʡ���Ƥ������ӡ����ͼ��ʾ�ı�ǩ�����ᵽһ�ɳ�ζ��
| ̼�����NH4HCO3 ���أ�50 kg ����������17.38% ������ѧ��ҵƷ��˾ |
����˸�����˵��
(1)���������______________�ʣ��ܴ�ʹ���ᆬҶ����ïʢ��ҶɫŨ�̣�ÿ�������к���Ԫ������Ϊ________________kg��
(2)�û���____________ʱ���ֽ⣬��ʩ��ʱ������__________�����ʻ��á�
 Na2CO3�� H2O �� CO2�������У�NaHCO3���� �����ᡱ��������Ρ�������Ӧ������ ��Ӧ��Na2CO3�������� ��
Na2CO3�� H2O �� CO2�������У�NaHCO3���� �����ᡱ��������Ρ�������Ӧ������ ��Ӧ��Na2CO3�������� ��