��Ŀ����
ˮ�DZ������Ȼ��Դ���ڹ�ũҵ�������ճ��������й㷺��Ӧ�ã�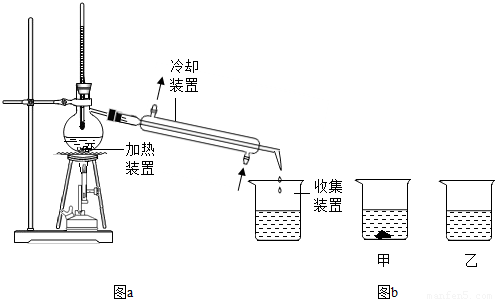
��1����ͼa�����־���ˮ�ķ����������ˡ�ˮ���и������ʵ� ��ͬ��������ĸ��ţ�
A���ܽ��� B���۵� C���е� D���ܶ�
��2��Ӳˮ��������������������㣬����������
��������Ӳˮ����ˮ��
��3��ˮ�Ǻܺõ��ܼ����±���KNO3��KCl�ڲ�ͬ�¶�ʱ���ܽ�ȣ���ش��������⣺
| �¶�/�� | 10 | 20 | 30 | 40 | 50 | 60 | 70 | ||
| �ܽ��/g | KNO3 | 13.3 | 20.9 | 31.6 | 45.8 | 63.9 | 85.5 | 110 | 138 |
| KCl | 27.6 | 31.0 | 34.0 | 37.0 | 40.0 | 42.6 | 45.5 | 48.3 | |
�ڲ�������һ�ֲ����������������ձ��е�ʣ�����ȫ���ܽ⣬������Һ��Ϊ��������Һ������˵������ȷ���� ������ĸ����
A���ܼ��������ܲ��� B����Һ�����ʵ���������һ����С
C����Һ����һ������ D����Һ�����ʵ�����������������
���𰸡������������ԭ���ǣ����ø���ֵķе㲻ͬ���з��룻�÷���ˮ��������Ӳˮ����ˮ����ĭ�������ˮ����ĭ�ٵ���Ӳˮ��KNO3���ܽ�ȱ�KCl���ܽ�����¶ȵ�Ӱ��仯��������ձ��е�����������أ����������ձ��е�ʣ�����ȫ���ܽ⣬������Һ��Ϊ��������Һ��˵����ȷ���ǣ�����ʱ���ܼ��������ܲ��䣬��Һ�����ʵ�������������������Һ����һ��������Һ�����ʵ������������ܼ�С��
����⣺��1�������ԭ���ǣ����ø���ֵķе㲻ͬ���з��룬�ʴ�Ϊ��C
��2���÷���ˮ��������Ӳˮ����ˮ����ĭ�������ˮ����ĭ�ٵ���Ӳˮ���ʴ�Ϊ������ˮ
��3�����������ձ��е�ʣ�����ȫ���ܽ⣬������Һ��Ϊ��������Һ��˵����ȷ���ǣ�����ʱ���ܼ��������ܲ��䣬��Һ�����ʵ�������������������Һ����һ��������Һ�����ʵ������������ܼ�С���ʴ�Ϊ������أ�ACD
�����������㿼���������ԭ����Ӳˮ����ˮ�����֡�������Һ�벻������Һ��ת��ȣ�֪ʶ��Ƚ϶࣬Ҫ��ǿ���⣬�ۺ�Ӧ�ã�
����⣺��1�������ԭ���ǣ����ø���ֵķе㲻ͬ���з��룬�ʴ�Ϊ��C
��2���÷���ˮ��������Ӳˮ����ˮ����ĭ�������ˮ����ĭ�ٵ���Ӳˮ���ʴ�Ϊ������ˮ
��3�����������ձ��е�ʣ�����ȫ���ܽ⣬������Һ��Ϊ��������Һ��˵����ȷ���ǣ�����ʱ���ܼ��������ܲ��䣬��Һ�����ʵ�������������������Һ����һ��������Һ�����ʵ������������ܼ�С���ʴ�Ϊ������أ�ACD
�����������㿼���������ԭ����Ӳˮ����ˮ�����֡�������Һ�벻������Һ��ת��ȣ�֪ʶ��Ƚ϶࣬Ҫ��ǿ���⣬�ۺ�Ӧ�ã�

��ϰ��ϵ�д�
 ��ĩ���䵥Ԫ�����ิϰ��ϵ�д�
��ĩ���䵥Ԫ�����ิϰ��ϵ�д�
�����Ŀ

 ��2012?��ɽ��һģ��ˮ�DZ������Ȼ��Դ���ڹ�ũҵ�������ճ��������й㷺��Ӧ�ã�
��2012?��ɽ��һģ��ˮ�DZ������Ȼ��Դ���ڹ�ũҵ�������ճ��������й㷺��Ӧ�ã�