��Ŀ����
����Ŀ����13���������д����л�ѧ��
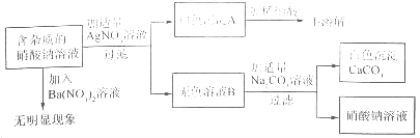
��һ�������������ʵ������գ�
��С�մ� �ڸɱ� �ۻ���̿ ����ʯ�� ������� ��̼���
��1���������˹�������� ����2�����������Ƽ����� ��
��3���������������������ļ��� ����4�����������Ϸʵ��� ��
��5�������ھ�������ˮ���� ����6�����������ͷ۵��� ��
�������γ��ô������������������Լ۱ȵõ���Խ��Խ�������ߵ�ϲ�����䷢�����ȿ���ʹ�����ͣ�Ҳ����ʹ��Ŀǰ�����ƹ�ʹ�õ��Ҵ����͡��������м��������Ҵ��͵õ��Ҵ����͡�
��1���Ҵ���C2H5OH���ڿ����г��ȼ�յĻ�ѧ����ʽΪ ��
��2�������Ҵ����͵�����˵���в���ȷ���� �����������
A���Ҵ����ڲ���������Դ B���Ҵ������Ǵ�����
C��ʹ���Ҵ����Ϳɼ��ٴ�����Ⱦ D��ʹ���Ҵ����Ϳɽ�ʡʯ����Դ
�������γǻ�����վ¥ʹ���˸ּܽṹ
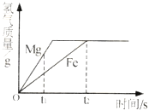
��1�������� ���������������
��2������ʴ����������е� ���ѧʽ�����������õĽ������ֹ������ʴ�ɲ��õķ����� ��д����һ������
��3����ͼ�У��ɱ�ʾ���������� ��

���𰸡���һ����1����
��2����
��3����
��4����
��5����
��6����
��������1��C2H5OH+3O2 ![]() 2CO2+3H2O��
2CO2+3H2O��
��2��AB
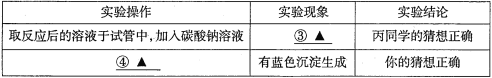
��������1������
��2��O2 H2O��ˢ��(����������Ҳ������
��3��c
��������
�����������һ����1���ڸɱ����������ȣ��������˹����ꣻ
��2����̼��ƺ��и�Ԫ���Ҷ����岻�����Σ���������ڲ��ƣ�
��3������ʯ���Ǽ���м������к����ԣ������ڸ�������������
��4��������غ���N��P��K�е�N��K�����Ǹ��Ϸʣ�
��5��������̿���������ԣ��ܳ�ɫ�غ���ζ��
��6����С�մ�����ֽ����������̼���Ҷ���������
��������1���ԣ�
��2��A���Ҵ���ͨ����ʳ���죬�ǿ�������Դ������B���Ҵ����ͺ����Ҵ������ͣ��ǻ�������C������ȼ�ղ���������Ⱦ���ʣ����Ҵ�ȼ�ղ���������̼��ˮ���ܲ���һ����̼��������Ҵ����沿�����Ϳɼ�����Ⱦ����ȷ��D�����沿�����ͣ���ȷ�����ϴ������AB��
��������1���������ĺϽ𣬻���
��2�������������������������ˮ�����Ĺ�ͬ���ã���ֹ����ɸ�����������ĽӴ����ָ���ȣ�����ԭ�ӹ��ɣ�ΪC��