��Ŀ����
����Ŀ��ij̼������Ʒ�к���һ������̼�����ơ�Ϊ�ⶨ�䴿�ȣ�ȡ100 g��Ʒ���������ٲ������壨2NaHCO3![]() Na2CO3 + H2O +CO2������ʹ���ɵ�ˮ��ȫ��Ũ�������գ���ü���ʱ���Ũ�����������ӵĹ�ϵ���±���
Na2CO3 + H2O +CO2������ʹ���ɵ�ˮ��ȫ��Ũ�������գ���ü���ʱ���Ũ�����������ӵĹ�ϵ���±���
ʱ�� ���ʵ����� | 0���� | 1���� | 2���� | 3���� | 4���� |
�����ͼ�ʯ�ҵ������ܺͣ�g�� | 150 | 150.9 | 151.8 | 152.7 | 152.7 |
��1������ȫ��Ӧ������ˮ������Ϊ______g
��2��������Ʒ��̼�����Ƶ����������Ƕ���____��
��3��������ȫ�ֽ��������ܽ���300 gˮ����ȫ�ܽ����������Һ�����ʵ���������____��
���𰸡�2.7 25.2% 23.2%
��������
��1���ɱ����֪����ˮ������Ϊ152.7g-150g=2.7g��
��2���裺�μӷ�Ӧ��̼�����Ƶ�����Ϊx ������̼���Ƶ�����Ϊy��
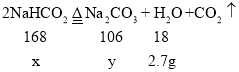
![]() x= 25.2 g
x= 25.2 g
![]() y =15.9g
y =15.9g
��Ӧ���ɵĶ�����̼����=25.2g-15.9g-2.7g=6.6g��
̼�����Ƶ���������=![]() ��
��
��4����Һ��̼���Ƶ���������=![]()

 ��ʦָ����ĩ��̾�ϵ�д�
��ʦָ����ĩ��̾�ϵ�д�����Ŀ��ijͬѧ���±��е��ܽ�����ݷ����ó��Ľ�����ȷ���ǣ�������
�¶�/�� | 0 | 20 | 40 | 60 |
KCl���ܽ��/g | 27.6 | 34.0 | 40.0 | 45.5 |
KNO3���ܽ��/g | 13.3 | 31.6 | 63.9 | 110 |
Ca��OH��2���ܽ��/g | 0.18 | 0.16 | 0.14 | 0.11 |
A.����ص��ܽ�����
B.���¿ɽ������͵�Ca��OH��2��Һ��Ϊ������Һ
C.40��ʱ�����͵��Ȼ�����Һ������������Ϊ40%
D.��KNO3�л�������KClʱ����������ȴ�ȱ�����Һ�ķ����ᴿ