��Ŀ����
�����еĸ�Ԫ����Ҫ�����ڹ����������У����ǻ������
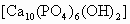
(1)
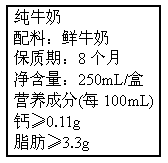
�ñ�ǩ����֬����3.3g����ָ100mLţ�̺�֬������������Ϊ3.3g����ôһ��ţ�̺�������________g��(��ȷ��0.01g)(2)
�ǻ�������и�Ԫ������Ԫ�ص��������Ƕ��٣�(3)
�ǻ�������и�Ԫ�ص����������Ƕ��٣�(��ȷ��0.01��)(4)
������ÿ��������Ҫ0.6g�ƣ�����Щ����90������ţ�̣���һ����ÿ������Ҫ��_______��ţ��.
�𰸣�0.28;200:93;39.8%;2
������
������
|
�⣺(1)������0.11g����ָ100mLţ�̺��Ƶ���������Ϊ0.11g����ô250mLţ�̺�������Ϊ�� (2) �ǻ�������и�Ԫ������Ԫ�ص��������ǣ�(40 ��10)��(31��6)=200��93��(3) �ǻ�������и�Ԫ�ص����������ǣ�
(4)0.6g ��90����0.28g������2�� |

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
�����еĸ�Ԫ����Ҫ�����ڹ����������У����ǻ�����ƾ���[Ca10��PO4��6��OH��2]��ʽ���ڣ�����Է�������Ϊ1004��ţ�̺��Ʒḻ�������գ���ţ�̵ĸƺ��ױ������ʣ��ǽ��ǵ�����ʳƷ����ͼ��ij��ҵ��˾��ţ�̰�װ��ǩ�IJ������֣�����ϸ�Ķ���ش��������⣺
��1����װ��ǩ��֬����3.3g����ָ100mLţ���У���֬������������Ϊ3.3g����ôһ��ţ�̺������� g��������0.01g����
��1����װ��ǩ��֬����3.3g����ָ100mLţ���У���֬������������Ϊ3.3g����ôһ��ţ�̺�������
| ��ţ�� ���ϣ���ţ�� �����ʣ�8���� ��������250mL/�� Ӫ���ɷ֣���ÿ100mL�� �ơ�0.11g ֬����3.3g �����ʡ�2.9g��2�����ǻ�������и�Ԫ�ص���������������Ϊ0.1%���� ��3��������ÿ��������Ҫ0.6g�ƣ�����Щ��90%����ţ�̣���һ����ÿ������Ҫ�ȶ��ٺ�ţ�̣� |
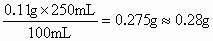 ��
��
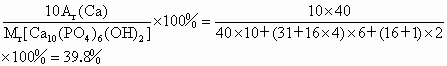
 �����еĸ�Ԫ����Ҫ�����ڹǸ�������У������ǻ�����ƾ���[Ca10��PO4��6��OH��2]��ʽ���ڣ�ţ�̺��Ʒḻ�������գ��ǽ��ǵ�����ʳƷ����ͼ��ij��ţ�̰�װ��ǩ�IJ������֣�����ϸ�Ķ��ش�
�����еĸ�Ԫ����Ҫ�����ڹǸ�������У������ǻ�����ƾ���[Ca10��PO4��6��OH��2]��ʽ���ڣ�ţ�̺��Ʒḻ�������գ��ǽ��ǵ�����ʳƷ����ͼ��ij��ţ�̰�װ��ǩ�IJ������֣�����ϸ�Ķ��ش� ��2009?�����������еĸ�Ԫ����Ҫ���ǻ�����ƾ���[Ca10��PO4��6��OH��2]��ʽ�����ڹ����������У���ͼ��ij��ҵ��˾��ţ�̰�װ��ǩ�IJ�������˵��������˵������ȷ���ǣ�������
��2009?�����������еĸ�Ԫ����Ҫ���ǻ�����ƾ���[Ca10��PO4��6��OH��2]��ʽ�����ڹ����������У���ͼ��ij��ҵ��˾��ţ�̰�װ��ǩ�IJ�������˵��������˵������ȷ���ǣ�������