��Ŀ����
����Ŀ���й��Ǻ˵������˵�վ�к�ȼ���˻��������ӵ�ײ���£�ԭ�Ӻ˷������ѣ���������믡��⡢嵐��ȵ�ԭ�Ӻ�һЩ���Ӽ����ߣ�ͬʱ�ͷŴ�������������Щ�����������������ֻ����硣
(1)�����ʵı仯�Ͽ����˱仯�뻯ѧ�仯����ͬ����_________________________��
(2)��ԭ�ӵı仯�Ͽ����˱仯�뻯ѧ�仯�IJ�ͬ����________________________��
(3)��ͼΪԪ�����ڱ�����Ԫ�ص������Ϣ������˵����ȷ����__________��
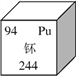
A�����Ƿǽ���Ԫ�� B����ԭ�Ӻ�����94������
C����Ԫ�ص�������94 D���е����ԭ������Ϊ244 g
���𰸡� �������µ����� �˱仯��ԭ�ӷ����仯����ѧ�仯��ԭ�Ӳ��� B
��������(1)�����ʵı仯�Ͽ����˱仯�뻯ѧ�仯����ͬ���Ƕ������µ����ʡ�(2)��ԭ�ӵı仯�Ͽ����˱仯�뻯ѧ�仯�IJ�ͬ���Ǻ˱仯��ԭ�ӷ����仯����ѧ�仯��ԭ�Ӳ����� (3)A�����ǽ���Ԫ�أ�����B��ԭ����������ֵ�ϵ���ԭ���ڵ���������ԭ����������=�������������ԭ�Ӻ�����94�����ӣ���ȷ��C����Ԫ�ص�������94������D���е����ԭ������Ϊ244������ѡB��

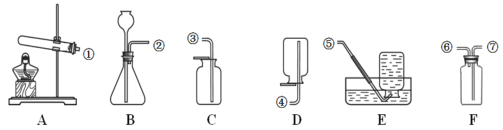
����Ŀ��(1)���Ʊ�������ƶԱ�ʵ����ʵ��̽������Ҫ������
ʵ��һ | ����Ӳˮ����ˮ |
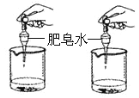
| ��ʵ����������Ҫ������ȡӲ����ˮ�������ͬ�⣬ ������Ҫ����_________________��ͬ�� |
ʵ��� | ̽��������Ŀ����������������ʲô��ͬ |
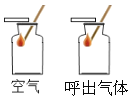
| ��ȼ�ŵ�Сľ���ֱ��������ͺ��������У��Աȹ۲죺ľ��ȼ��ʱ��ij��̡� ���Եó��Ľ�����_____________�� |
ʵ���� | ̽����Һ�е����� |
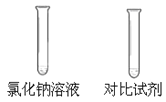
| ��֤�������ܵ��磬ˮ�����ѵ��硣 ��ѡ��_________���Ա��Լ��� |
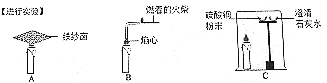
(1)�۲���ѧϰ��ѧ����Ҫ������
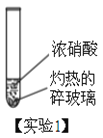
�о�С���������ʵ�飬���־��к���ɫ�����������������
|
|
|
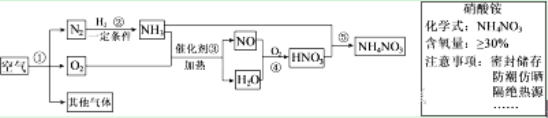
�������ϣ�4HNO3 ![]() O2�� + 4NO2�� + 2H2O C + 4HNO3(Ũ)
O2�� + 4NO2�� + 2H2O C + 4HNO3(Ũ) ![]() CO2�� + 4NO2�� + 2H2O
CO2�� + 4NO2�� + 2H2O
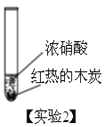
�٣�ʵ��1���������鲣����Ũ���������ѧ��Ӧ�����ȵ��鲣����ʵ������������___��
�ڣ�ʵ��2�����к���ɫ����������ܷ�֤����ʵ��2����ľ̿��Ũ���ᷢ���˷�Ӧ���������жϣ���˵�����ɣ�________��
�ۣ�ʵ��3���з������ɵ���������м���������̼ (�����������0.03% )���ܷ�֤����ʵ��3����ľ̿��Ũ���ᷢ���˷�Ӧ���������жϣ���˵�����ɣ�_________��