��Ŀ����
ˮ����Һ���������������������ء���������ͼʾ����ش�������⡣
ͼ1����̿��ˮ�� ͼ2 ˮ�ĵ��
ͼ2 ˮ�ĵ��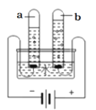
ͼ3 �ܽ������ ͼ4 ̽��ʵ��
ͼ4 ̽��ʵ��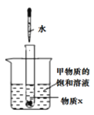
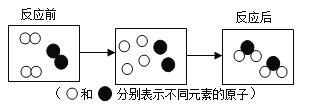
��1��ͼ1�У�����̿��ˮ����Ҫ�����˻���̿���е�____�ԣ�ͼ2��ʾʵ���У��Թ�b�еõ��������ǣ�д�������ƣ�____��
��2��ͼ3Ϊ�ס������ֹ������ʵ��ܽ������ͼ��t1��ʱ�������ʵ��ܽ����__��t2��ʱ���ı�����Һ������������_________�ҵı�����Һ������������������ڡ��������ڡ�����С�ڡ�֮һ����ͼ4��ʾʵ���У���ˮ����ʢ������X��С�Թ��У��ɹ۲쵽�ձ����м����ʵĹ���������������X������_________�������֮һ����
A ʳ�� B �ռ� C ��ʯ�� D �����
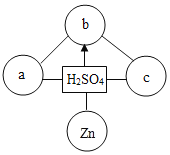
��3����ͼ���Ȼ��ƺ�������ڲ�ͬ�¶ȵ��ܽ�ȣ���ش�
�¶�/�� | 0 | 20 | 40 | 60 | 80 | 100 | |
�ܽ��/g | KNO3 | 13.3 | 31.6 | 63.9 | 110 | 169 | 246 |
NaCl | 35.7 | 36.0 | 36.6 | 37.3 | 38.4 | 39.8 |
��20��ʱ����136g����NaCl��Һ����10gˮ���ٽ��µ�20�棬������NaCl���������Ϊ____��
��ijС������20%���Ȼ�����Һ���ܶ�Ϊ1.17g/cm3��,����30g��������Ϊ10%���Ȼ�����Һ����Ҫ20%���Ȼ�����Һ____mL������������ȷ��0.1��
�������ϱ�����������ϵ�л��Ƴ�NaCl��KNO3���ܽ�����ߣ��������ߵĽ����Ӧ���¶ȷ�Χ��___������ţ���
A 0��20�� B 20��40�� C 40��60�� D 60��80��
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�