��Ŀ����
����Ŀ��ע������һ����ͨ��ҽ����е�������ڻ�ѧʵ���з�����Խ��Խ������á�ij��ѧ��ȤС��Ϊ�˲ⶨ�����������ĺ���������������̽�����
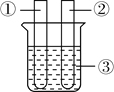
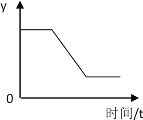
���������ϣ���1�������Ż����40�棬�����Ż����240�棬�������ʵ�ȼ�ղ��������������ǰ�ɫ���壬��̼��������������������е�ˮ������Ӧ�������ж���ƫ����![]() ��
��
��2��ͭ˿�ڼ��������¿���������е�������Ӧ���ɹ�̬������ͭ��
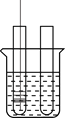
�����ʵ�飩�������ͼ��װ�ý���̽�����
������������
��1�������Թܵ��ݻ�Ϊ![]() ��
��
��2�����װ�õ����������ã�
��3��װҩƷ����ע������������������λ�ã����Ӻ�������
��4���õ��ɼмн���Ƥ�ܣ����ȴ�ͭ˿���۲�����
��5��ȼ�ս��������ɼУ��۲�ע�����������ƶ������
����˼�뽻����
��1��ʵ����������ͭ˿��______�ԣ��÷������ŵ���_________��
��2����ȤС��ͬѧ�����Թ��ݻ��ķ���������________��
��3�����裨3����ע������������Ӧ����______mL��������������
��4�����裨4���й۲쵽�������У�______________��
��5��������ղ����Ľ��ƫС���ܵ�ԭ���У�___________��
��6���÷�Ӧ�����ֱ���ʽΪ___________��
��7����ͬѧ��Ϊͭ˿���������������ʲ��������ȷ��______��ͬ�⡢��ͬ�⣩�������ǣ�________________��
���𰸡����� ʼ���ܱգ����� װ��һ�Թ�ˮ��������Ͳ�в���ˮ����� 10 ����ȼ�գ������������� ���ײ��㣻δ��ȴ�ʹ��ɼ� ���ף����� ![]() ���������� ��ͬ�� ͭ˿����������������װ��ѹǿƫС
���������� ��ͬ�� ͭ˿����������������װ��ѹǿƫС
��������
��1������ʵ��װ�ã����Թ��ⲿ����ͭ˿����Ϊ����ȼ���ף�������ͭ���õĵ��������÷������ŵ���ʼ���ܱգ�������
��2�������Թ��ݻ��ij��÷����ǣ����Թ�װ��ˮ��Ȼ�����ˮ�������
��3������Լռ������������֮һ���Թܵ��ݻ�Ϊ50mL�����Թ�5�ȷ֣�ÿһ�ȷ�Ϊ10mL���ʻ�������Ӧ������10mL����
��4�����ȴ�ͭ˿���۲����ῴ������ȼ�գ����ƹ⡢���ȡ�ð�������İ�����
��5��������ղ����Ľ��ƫС���ܵ�ԭ���а��ײ��㡢δ��ȴ�ʹ��ɼУ�
��6������ȼ���������������ף��÷�Ӧ�����ֱ���ʽΪ���ף����� ![]() ���������ף�
���������ף�
��7��ͭ˿����������������ʹ�������ĸ���֣���������װ��ѹǿƫС������Ӱ��ʵ�������ʲ�ͬ���˵����

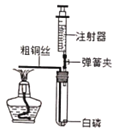
����Ŀ��С��ͬѧ����±���ʾʵ�飬̽������ͭ��H2O2�ֽ����ʵ�Ӱ�죮
���� | װ�� | ���� |
��1��ȡһ��ͭ˿��������Ͳ�Σ��̶�����˿�ϣ� |
| |
��2���ֱ���ٺ͢���ע��15mL��30%��H2O2��Һ��������ʢ����ˮ�Ģ��С� |
| Լ1min��ɹ۲쵽�ٺ͢��ж����������ݲ����� |
��3����ͭ˿������ͬ�̶�����˿��������С� |
| �����д������ݲ�����5min���������ݲ�������ʱ������Ȼ�������������ݲ����� |
�ش�����������
��1����������ʵ���Ŀ����________��
��2���ܷ�ó�ͭ���Լӿ�H2O2�ֽ����ʵĽ���________����ǡ�����
��3�����������ʵ��̽��ͭ�Dz���H2O2�ֽ�Ĵ�������Ҫ������ʵ�鷽�����в��䣬���б�Ҫ����________��
A������ʵ��ǰͭ˿����������ʵ������ͭ˿��������
B��������������������
C����ʵ����ͭ˿�����м���
D������˿����ͭ˿
E��������������Һ��ˮϡ��
��4��ʵ���ȷ��ͭ���Լӿ�H2O2�ֽ�����ʣ���д���÷�Ӧ�Ļ�ѧʽ����ʽ_______________��