��Ŀ����
����Ŀ��ʳ�������������г��������ʣ����Ź㷺����;��
��1����ˮ�к��н϶���Ȼ��ƣ��ú�ˮɹ�ο��Ի��ʳ�Ρ��Ȼ����ں�ˮ�еĴ�����ʽ�� _____(�ѧ����)��
��2����ˮɹ���ƵõĴ����к���һЩ��ɳ����ȥ��ɳ�������������Ի�ýϴ����Ȼ��Ƶ�ʵ�鷽��Ϊ:�Ƚ���������һ������ˮ�У�____________��
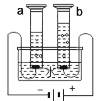
��3����Ҫ����100 g������������Ϊ2%��ʳ��ˮ�����Ƶ�ʵ�鲽����Ը���Ϊ���㡢������_______��ת�Ƽ�����ǩ���������֪��Ӧ��������ƽ��ȡ_______g�Ȼ��ƣ�Ӧѡ�ù��Ϊ_________mL����Ͳ����ȡˮ��
��4��ʵ������������ʧȥ��ǩ���Լ�ƿ��һƿװ��ʳ��ˮ��һƿװ������ˮ����д�����ַ�������������(Ҫ˵������):��________;��__________��
��5��ҽ��ʹ�õ�������ˮ��������������ԼΪ0. 9%����Ҫ����500 g 0. 9%��������ˮ������Ҫ��_____g������������Ϊ2%��ʳ��ˮ��ϡ�͡�
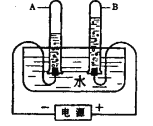
��6����ҵ���õ�ⱥ��ʳ��ˮ�ķ����������ռ�(NaOH)������(Cl2 )��ͬʱ���õ���һ�ֵ������壬д���÷�Ӧ�Ļ�ѧ����ʽ:_______��
���𰸡�Na+��Cl- Ȼ����ˡ����� �ܽ� 2 100 �ֱ�μ���������Һ���Ȼ���������������Һ��Ӧ�����Ȼ�����ɫ�������а�ɫ���������ɵ���ʳ��ˮ��û�е�������ˮ �ֱ�ⶨ�����ԣ�������ǿ����ʳ��ˮ���������������ˮ 225 2NaCl+2H2O![]() 2NaOH+Cl2��+H2��
2NaOH+Cl2��+H2��
��������
��1���Ȼ������������Ӻ������ӹ��ɵģ������Ȼ����ں�ˮ�еĴ�����ʽ��Na+��Cl-��
��2�������ᴿ�IJ����ǣ��ܽ⡢���ˡ����������Գ�ȥ��ɳ�������������Ի�ýϴ����Ȼ��Ƶ�ʵ�鷽��Ϊ���Ƚ���������һ������ˮ�У�Ȼ����ˡ�������
��3������һ������������Һ�IJ����ǣ����㡢�������ܽ⡢װƿ����������=��Һ����������������������Ͳʹ��ʱ�����þͽ�ԭ������Ӧ��������ƽ��ȡ�Ȼ�������Ϊ��100g��2%=2g��ˮ������Ϊ��100g-2g=98g����98mL��Ӧѡ�ù��Ϊ100mL����Ͳ����ȡˮ��
��4���ֱ�μ���������Һ���Ȼ���������������Һ��Ӧ�����Ȼ�����ɫ�������а�ɫ���������ɵ���ʳ��ˮ��û�е�������ˮ���ֱ�ⶨ�����ԣ�������ǿ����ʳ��ˮ���������������ˮ��ʳ��ˮ��ʳ�ε�ˮ��Һ�������������ܼ��������о�����������ʳ��ˮ��û�е�������ˮ��
��5����Һϡ��ǰ�������������䣬����Ҫ����500g0.9%��������ˮ������Ҫ��![]() =225g������������Ϊ2%��ʳ��ˮ��ϡ�ͣ�
=225g������������Ϊ2%��ʳ��ˮ��ϡ�ͣ�
��6���Ȼ��ƺ�ˮ��ͨ��������������������ơ���������������ѧ����ʽΪ��2NaCl+2H2O![]() 2NaOH+Cl2��+H2����
2NaOH+Cl2��+H2����

 ��ĩ���100�ִ��½����ȫ�Ծ�ϵ�д�
��ĩ���100�ִ��½����ȫ�Ծ�ϵ�д�����Ŀ��Ԫ�����ڱ���ѧϰ���о���ѧ����Ҫ���ߣ������Ԫ�����ڱ���һ����
��һ���� | 1 H 1.008 | 2 He 4.003 | ||||||
�ڶ����� | 3 Li 6.941 | 4 Be 9.012 | �� | 6 C 12.01 | 7 N 14.01 | 8 O 16.00 | 9 F 19.00 | 10 Ne 20.81 |
�������� | �� | 12 Mg 24.31 | 13 Al 26.98 | 14 Si 28.09 | 15 P 30.97 | 16 S 32.06 | �� | 18 Ar 39.95 |
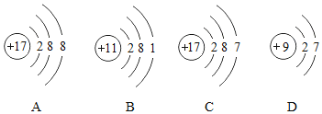
��1��ѧϰ��ѧ������ѧ���˴��۽Ƕ���ʶ�����������ͼ�������ӵĽṹʾ��ͼ���ش����⣺
����������ͬ��Ԫ�ص���_____������ţ���ͬ��
��ͼC��ijԪ�ص�ԭ�ӽṹʾ��ͼ����Ԫ����Ԫ�����ڱ��е�λ����_____��ѡ��ٻ�ڻ�ۣ�
��2��ԭ������Ϊ12��Ԫ�ص�ԭ���ڻ�ѧ��Ӧ����_____����á���ʧ�������ӣ��γ����ӵķ�����_____����Ԫ���ڻ������еĻ��ϼ�Ϊ_____�ۣ���Ԫ�غ�ԭ������Ϊ9��Ԫ����ɵĻ�������_____���ѧʽ����
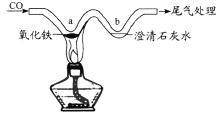
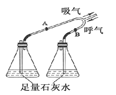
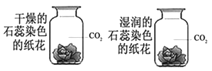
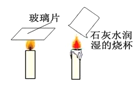
��3��̽����������ˮ��Ӧʱ���������������ɣ��ռ����岢��ȼ�������Ա���������Ӧ����Һ�еμӷ�̪�Լ�����Һ�ʺ�ɫ���ɴ��ƶϣ�����ˮ��Ӧ�Ļ�ѧ����ʽΪ_________����Ӧ����������_________��
����Ŀ����1���±�����������ˮ���ұ��IJ������ݡ�
��������ˮˮ�ʳ��������Ŀ����ֵ�����֣�
��Ŀ | ��ֵ |
ɫ�� | ������15�ȣ������ó���������ɫ |
���Ƕ� | ������1�ȣ���������²�����5�� |
pH | 6.5��8.5 |
��Ӳ�ȣ���CaCO3�ƣ� | 450mg/L |
�� | 0.2mg/L |
�� | 0.3mg/L |
������ | 250mg/L |
���ϱ���������ָ����_______��ѡ������������Ԫ��������ԭ����)��
��������Ӳˮ�����ķ�����___________��
��Cl2��ClO2����������ˮ��������������Ԫ�صĻ��ϼ۷ֱ���____________��
��1L�ϸ����������ˮ�У�����CaCO3����Ӳ�ȣ����Ԫ�صĺ���������_____mg/L��
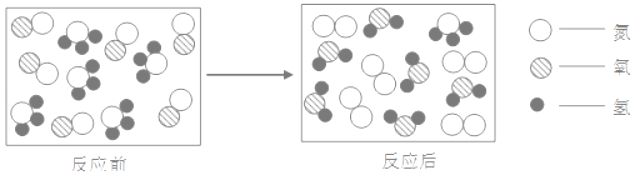
��ʵ����������ˮ��ͨ��ֽ⣬���������������������ԼΪ______����Ӧ�Ļ�ѧ����ʽΪ______��
��2��Ԫ�����ڱ���ѧϰ��ѧ����Ҫ���ߡ�������Ԫ�����ڱ���1��18��Ԫ��ԭ�Ӻ�������Ų���
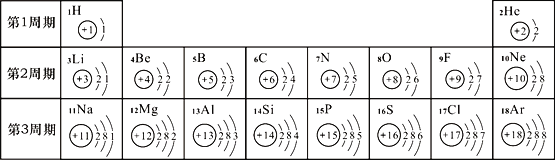
��ش��������⣺
����17��Ԫ������______Ԫ��(���������������ǽ�����)�������������Ϊ______��
��Ԫ�صĻ�ѧ������ԭ�ӽṹ�е�_____����ϵ���У���ͬһ���У���Ԫ�ص�ԭ�ӽṹ���ֵĹ�����____����дһ�㣩��
�۵ؿ��к������Ľ���Ԫ���뺬�����ķǽ���Ԫ����ɵĻ�����Ϊ____________����16��Ԫ���ڻ�ѧ��Ӧ�������γ�________�������ӷ��ţ�����Ԫ�صĵ�����������ȼ�յ�������_______________��