��Ŀ����
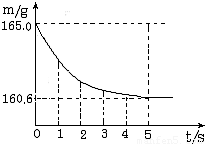
���ձ��м����Ȼ��ƺ�̼�����ƵĹ�������10.0g���ټ���68.9gϡ����ǡ����ȫ��Ӧ����Ӧ�����þ�����������ձ���ͬҩƷ����������m���뷴Ӧʱ�䣨t���Ĺ�ϵ��ͼ��ʾ���ձ���ͬҩƷ����ʼ������Ϊ165.0g����Ӧ�ķ���ʽΪ��NaHCO3+HCl�TNaCl+H2O+CO2���Իش��������⣺��1����ȫ��Ӧʱ����������̼������Ϊ______g
��2��ԭ��������Ȼ��Ƶ�������
��3����Ӧ�������Ȼ�����Һ����������������
���𰸡���������1���Ȼ��ƺ�̼�����ƵĹ���������ϡ�����Ϻ�̼�����������ᷴӦ�����Ȼ��ơ�ˮ�Ͷ�����̼�����ڷ�Ӧ�ų��˶�����̼�����Է�Ӧ���ձ���ʣ�����ʵ�������С����С��������Ϊ���������̼��������
��2�����ݷ�Ӧ�Ļ�ѧ����ʽ�������������������̼�������ɼ���ԭ�������̼�����Ƶ��������������������������̼�����Ƶ������Ϊ��������Ȼ��Ƶ�������
��3��ǡ����ȫ��Ӧ��������ҺΪ�Ȼ�����Һ�����������Ȼ����ɻ�������Ȼ��ƺͷ�Ӧ���ɵ��Ȼ�������ɣ�
��ʱ������Һ���Ȼ��Ƶ���������= ×100%�����ݷ�Ӧ�������Ӧ���ɵ��Ȼ������������������غ���㷴Ӧ����Һ��������
×100%�����ݷ�Ӧ�������Ӧ���ɵ��Ȼ������������������غ���㷴Ӧ����Һ��������
����⣺��1�����������غ㶨�ɣ���Ӧ���ɶ�����̼���������=165.0g-160.6g=4.4 g
��2����ԭ�������̼�����Ƶ�����Ϊx�������Ȼ��Ƶ�����Ϊy
NaHCO3+HCl=NaCl+H2O+CO2��
84 58.5 44
x y 4.4g

��֮�ã�x=8.4g��y=5.85g
����ԭ��������Ȼ��Ƶ�����Ϊ10.0g-8.4g=1.6g
��2����Ӧ��������Һ����=68.9 g+10.0g-4.4g=74.5g
��Ӧ��������Һ��������������=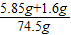 ×100%=10%
×100%=10%
�𣺷�Ӧ��������Һ��������������10%��
�ʴ�Ϊ����1��4.4����2����ԭ��������Ȼ��Ƶ�����Ϊ1.6g��3���𣺷�Ӧ�������Ȼ�����Һ����������������10%��
������������Ҫ����ѧ�����û�ѧ����ʽ����������������ʽ���м��������������ʱҪ�ر�ע����ⲽ��Ĺ淶�ԣ��Ӷ�����ͨ����֪����δ֪�Ľ��ⷽ����
��2�����ݷ�Ӧ�Ļ�ѧ����ʽ�������������������̼�������ɼ���ԭ�������̼�����Ƶ��������������������������̼�����Ƶ������Ϊ��������Ȼ��Ƶ�������
��3��ǡ����ȫ��Ӧ��������ҺΪ�Ȼ�����Һ�����������Ȼ����ɻ�������Ȼ��ƺͷ�Ӧ���ɵ��Ȼ�������ɣ�
��ʱ������Һ���Ȼ��Ƶ���������=
 ×100%�����ݷ�Ӧ�������Ӧ���ɵ��Ȼ������������������غ���㷴Ӧ����Һ��������
×100%�����ݷ�Ӧ�������Ӧ���ɵ��Ȼ������������������غ���㷴Ӧ����Һ������������⣺��1�����������غ㶨�ɣ���Ӧ���ɶ�����̼���������=165.0g-160.6g=4.4 g
��2����ԭ�������̼�����Ƶ�����Ϊx�������Ȼ��Ƶ�����Ϊy
NaHCO3+HCl=NaCl+H2O+CO2��
84 58.5 44
x y 4.4g

��֮�ã�x=8.4g��y=5.85g
����ԭ��������Ȼ��Ƶ�����Ϊ10.0g-8.4g=1.6g
��2����Ӧ��������Һ����=68.9 g+10.0g-4.4g=74.5g
��Ӧ��������Һ��������������=
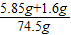 ×100%=10%
×100%=10% �𣺷�Ӧ��������Һ��������������10%��
�ʴ�Ϊ����1��4.4����2����ԭ��������Ȼ��Ƶ�����Ϊ1.6g��3���𣺷�Ӧ�������Ȼ�����Һ����������������10%��
������������Ҫ����ѧ�����û�ѧ����ʽ����������������ʽ���м��������������ʱҪ�ر�ע����ⲽ��Ĺ淶�ԣ��Ӷ�����ͨ����֪����δ֪�Ľ��ⷽ����

��ϰ��ϵ�д�
 �������Ͽ��㱾ϵ�д�
�������Ͽ��㱾ϵ�д�
�����Ŀ
a��ʵ���������Ȼ�þ�������ƵĹ���������Ʒ��С��ͬѧ��ⶨ��Ʒ���Ȼ�þ�������������ȳ�ȡ�û������Ʒ20g����ȫ����ˮ�У�Ȼ��ȡ����һ��������������������������Һ100gƽ�����Ĵμ������У������ʵ���������ݼ��±�����������������йؼ��㣺
��1���ϱ���X����ֵΪ ��
��2������20g����������Ʒ���Ȼ�þ��������
��3����������ʵ�������õ�������������Һ����������������
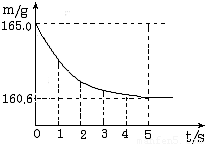
b�����ձ��м����Ȼ��ƺ�̼�����ƵĹ�������10.0g���ټ���68.9gϡ����ǡ����ȫ��Ӧ��
��Ӧ�������þ�����������ձ���ͬҩƷ����������m���뷴Ӧʱ�䣨t���Ĺ�ϵ��ͼ��ʾ���ձ���ͬҩƷ����ʼ������Ϊ165.0g����Ӧ�ķ���ʽΪNaHCO3+HCl=NaCl+H2O+CO2��������
��1����ȫ��Ӧʱ����������̼�������� ��
��2������ԭ��������Ȼ��Ƶ�������
��3������ϡ�������������������
| �������ʵ����� | 1 | 2 | 3 | 4 |
| ��������������Һ������/g | 25 | 25 | 25 | 25 |
| ���ɳ���������/g | 2.9 | X | 8.7 | 8.7 |
��2������20g����������Ʒ���Ȼ�þ��������

��3����������ʵ�������õ�������������Һ����������������
b�����ձ��м����Ȼ��ƺ�̼�����ƵĹ�������10.0g���ټ���68.9gϡ����ǡ����ȫ��Ӧ��
��Ӧ�������þ�����������ձ���ͬҩƷ����������m���뷴Ӧʱ�䣨t���Ĺ�ϵ��ͼ��ʾ���ձ���ͬҩƷ����ʼ������Ϊ165.0g����Ӧ�ķ���ʽΪNaHCO3+HCl=NaCl+H2O+CO2��������
��1����ȫ��Ӧʱ����������̼��������
��2������ԭ��������Ȼ��Ƶ�������
��3������ϡ�������������������
a��ʵ���������Ȼ�þ�������ƵĹ���������Ʒ��С��ͬѧ��ⶨ��Ʒ���Ȼ�þ�������������ȳ�ȡ�û������Ʒ20g����ȫ����ˮ�У�Ȼ��ȡ����һ��������������������������Һ100gƽ�����Ĵμ������У������ʵ���������ݼ��±�����������������йؼ��㣺
��1���ϱ���X����ֵΪ______��
��2������20g����������Ʒ���Ȼ�þ��������
��3����������ʵ�������õ�������������Һ����������������
b�����ձ��м����Ȼ��ƺ�̼�����ƵĹ�������10.0g���ټ���68.9gϡ����ǡ����ȫ��Ӧ��
��Ӧ�������þ�����������ձ���ͬҩƷ����������m���뷴Ӧʱ�䣨t���Ĺ�ϵ��ͼ��ʾ���ձ���ͬҩƷ����ʼ������Ϊ165.0g����Ӧ�ķ���ʽΪNaHCO3+HCl=NaCl+H2O+CO2��������
��1����ȫ��Ӧʱ����������̼��������______��
��2������ԭ��������Ȼ��Ƶ�������
��3������ϡ�������������������

| �������ʵ����� | 1 | 2 | 3 | 4 |
| ��������������Һ������/g | 25 | 25 | 25 | 25 |
| ���ɳ���������/g | 2.9 | X | 8.7 | 8.7 |
��2������20g����������Ʒ���Ȼ�þ��������
��3����������ʵ�������õ�������������Һ����������������
b�����ձ��м����Ȼ��ƺ�̼�����ƵĹ�������10.0g���ټ���68.9gϡ����ǡ����ȫ��Ӧ��
��Ӧ�������þ�����������ձ���ͬҩƷ����������m���뷴Ӧʱ�䣨t���Ĺ�ϵ��ͼ��ʾ���ձ���ͬҩƷ����ʼ������Ϊ165.0g����Ӧ�ķ���ʽΪNaHCO3+HCl=NaCl+H2O+CO2��������
��1����ȫ��Ӧʱ����������̼��������______��
��2������ԭ��������Ȼ��Ƶ�������
��3������ϡ�������������������

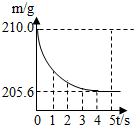 ���ձ��м����Ȼ��ƺ�̼���ƵĹ�������10.9g���ټ���113.5gϡ����ǡ����ȫ��Ӧ����Ӧ�����þ�����������ձ���ͬҩƷ����������m���뷴Ӧʱ�䣨t���Ĺ�ϵ��ͼ��ʾ��
���ձ��м����Ȼ��ƺ�̼���ƵĹ�������10.9g���ټ���113.5gϡ����ǡ����ȫ��Ӧ����Ӧ�����þ�����������ձ���ͬҩƷ����������m���뷴Ӧʱ�䣨t���Ĺ�ϵ��ͼ��ʾ��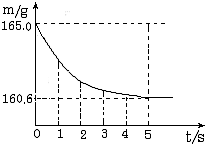 ��2005?�����ձ��м����Ȼ��ƺ�̼�����ƵĹ�������10.0g���ټ���68.9gϡ����ǡ����ȫ��Ӧ����Ӧ�����þ�����������ձ���ͬҩƷ����������m���뷴Ӧʱ�䣨t���Ĺ�ϵ��ͼ��ʾ���ձ���ͬҩƷ����ʼ������Ϊ165.0g����Ӧ�ķ���ʽΪ��NaHCO3+HCl�TNaCl+H2O+CO2���Իش��������⣺
��2005?�����ձ��м����Ȼ��ƺ�̼�����ƵĹ�������10.0g���ټ���68.9gϡ����ǡ����ȫ��Ӧ����Ӧ�����þ�����������ձ���ͬҩƷ����������m���뷴Ӧʱ�䣨t���Ĺ�ϵ��ͼ��ʾ���ձ���ͬҩƷ����ʼ������Ϊ165.0g����Ӧ�ķ���ʽΪ��NaHCO3+HCl�TNaCl+H2O+CO2���Իش��������⣺