��Ŀ����
����Ŀ����2014�������������˻��ϣ��й��˶�Ա������˾�ö���ƣ���Ϊ������̳������
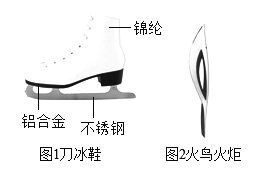
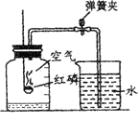
��1��ͼ1���ٶȻ����˶�Աʹ�õĵ���Ь��
�ٵ���Ь�к��еĽ���������______��д��ѧʽ��һ�ּ��ɣ�
�����쵶��Ь�����Ͻ���Ͼ��нϺõĿ���ʴ�ԣ����û�ѧ����ʽ��ʾ�����нϺõĿ���ʴ�Ե�ԭ��_______��
��2��ͼ2��2014���������»�Ļ�棬�����������˵�еĻ��ɼ�ʥ����̫�����½����ȼ����������澵����λ�ã����ţ���潫����ȼ������ܱ���ȼ��ԭ����___����洫�ݽ������ùر�ȼ�����ŵķ���Ϩ���棬�����̺������ԭ����_________��
���𰸡�Al����Fe�� 4Al+3O2�T2Al2O3 �¶ȴﵽ�Ż�� �����ȼ��
��������
��1���ٴӵ���Ь����ɲ��Ͽ�����Ь�к��еĽ����������������������л��ϳɲ��ϵ��ǽ��ڣ�
������ͨ������º�������Ӧ�Ļ�ѧ����ʽΪ��4Al+3O2�T2Al2O3��
��2�����澵�ܹ��۹⣬�۹�ʱ�¶����ߣ����ﵽȼ�ϵ��Ż��ʱ��ȼ���ܹ�ȼ�������������澵��ȼ��ȼ������������ʹ�¶ȴﵽȼ�ϵ��Ż�㣻�ر�ȼ�����ź�ȼ���������������Ӷ�Ϩ����

����Ŀ����һ���ܱ������з���A��B��C��D�������ʣ���һ�������·�����ѧ��Ӧ��һ��ʱ�����й��������£�
���� | A | B | C | D |
��Ӧǰ���� (g) | 9 | 2 | 22 | 1 |
��Ӧ������(g) | x | 2 | 6 | 21 |
(1)��һ��Ӧ�� x=________g��
(2)д��һ����������Ӧ������ͬ�Ļ�ѧ����ʽ��_________��
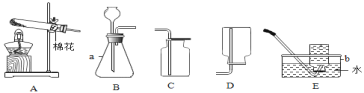
����Ŀ�����A��B����Ȥζʵ������ѡ1����������������𣬰�A�Ʒ֡�
A | B |
��1����һʢ�������ɱ��IJ��������м���������ˮ����������Ũ��İ����������������ԭ���ǡ�____ ��2����ȡһʢ����������ʯ��ˮ�IJ��������������м��������ɱ����۲쵽����ʯ��ˮ����ǣ�������Ӧ�Ļ�ѧ����ʽΪ______�� |
��1����ȼ������ӳ�ʾ����С�������С������____������ڡ������ڡ���С�ڡ���ȼ�պ����������������
��2���ò�����Ѹ�ٿ�סȼ�յ�����ʹ����ʼ�ս�û��ˮ�����ձ���Һ��____������ڡ������ڡ����ڡ�������Һ�档 |