��Ŀ����
����Ŀ�����A��B����Ȥζʵ������ѡ1����������������𣬰�A�Ʒ֡�
A | B |
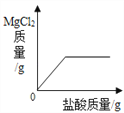
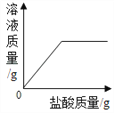
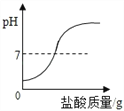
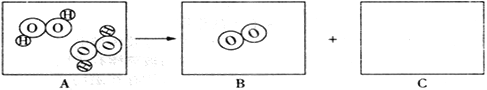
��1����һʢ�������ɱ��IJ��������м���������ˮ����������Ũ��İ����������������ԭ���ǡ�____ ��2����ȡһʢ����������ʯ��ˮ�IJ��������������м��������ɱ����۲쵽����ʯ��ˮ����ǣ�������Ӧ�Ļ�ѧ����ʽΪ______�� |
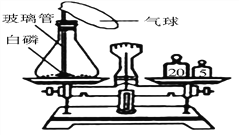
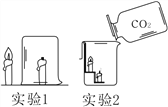
��1����ȼ������ӳ�ʾ����С�������С������____������ڡ������ڡ���С�ڡ���ȼ�պ����������������
��2���ò�����Ѹ�ٿ�סȼ�յ�����ʹ����ʼ�ս�û��ˮ�����ձ���Һ��____������ڡ������ڡ����ڡ�������Һ�档 |
���𰸡� �ɱ��������ȣ�ʹ��Χ��ˮ����������Сˮ�� CO2+Ca(OH)2CaCO3��+H2O С�� ����
����������1���ɱ��ǹ��������̼���׳ƣ���һʢ�������ɱ��IJ��������м���������ˮ����������Ũ��İ����������������ԭ���Ǹɱ��������ȣ�ʹ��Χ��ˮ����������Сˮ�Σ��γɰ�����(2). ������̼����ʯ��ˮ�е������������Ʒ�Ӧ����̼��Ƴ�����ˮ��ʹ����ʯ��ˮ����ǣ���Ӧ����ʽΪ��CO2+Ca(OH)2CaCO3��+H2O��(3)�������غ㶨�ɿ�֪���μӷ�Ӧ�ĸ����ʵ������ܺ͵��ڷ�Ӧ�����ɵĸ����ʵ������ܺͣ�����������ٵ������뷴Ӧ�������������ͣ���������������������ò�����Ѹ�ٿ�סȼ�յ�����ʹ����ʼ�ս�û��ˮ�����ձ���Һ���������Һ�棬��Ϊ����ȼ�����ĵ����ڵ����������ɵĶ�����̼������ˮ��ʹ����������٣�ѹǿ��С��С��������ѹ����ѹ����������£����е�Һ����뱭�ڡ�

 ���ſ����ϵ�д�
���ſ����ϵ�д� ���Ŀ����ϵ�д�
���Ŀ����ϵ�д� ������ӱ������ͯ������ϵ�д�
������ӱ������ͯ������ϵ�д� A�ӽ��� ϵ�д�
A�ӽ��� ϵ�д�