��Ŀ����
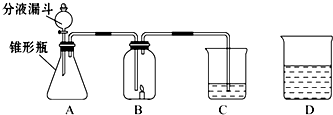 С����С�������������װ����ʵ�飬A�����ӵ�ҩƷ��ͬ��C������ҩƷ��ͬ��B�ж���һ��ȼ�ŵ�������ʱ�����ܳ���ȼ�գ�D��ʢ�ŵ���C��������Һ��
С����С�������������װ����ʵ�飬A�����ӵ�ҩƷ��ͬ��C������ҩƷ��ͬ��B�ж���һ��ȼ�ŵ�������ʱ�����ܳ���ȼ�գ�D��ʢ�ŵ���C��������Һ��
��1��С���ڷ�Һ©������ƿ��C�зֱ����ҩƷ����ʹ֮��ϣ��ٸ���B�����ӣ����ӳ���ͼ��ʾ������B�е�����Ϩ��C�е���Һ����ǣ�д��A�з�����Ӧ�����ֱ���ʽ______��C�з�Ӧ�����ֱ���ʽΪ______��
��2��С��Ҳ�ڷ�Һ©������ƿ��C�зֱ����ҩƷ����ʹ֮��ϣ��ٸ���B�����ӣ����ӳ���ͼ��ʾ������B�е�����ȼ�յø�����C�е���ҺҲ����ǣ�A�з�����Ӧ�����ֱ���ʽΪ______��С��˵C�е�����֤�������к���̼Ԫ�أ�����ΪС��˵�Ķ���______�����������______��
��3��С�����һ��̽��C�а�ɫ������ijɷ֣��Ͱ�C�еİ�ɫ�������ˮ��Һ�з��������С���õķ�����______��
��4���¿�ʱ����λͬѧ������û�����D��Һ������һ���Ĥ���ð�Ĥ�Ļ�ѧ�ɷֵ�������______��
��2����ʹ����ȼ�յø�����������������˵��A�з�Ӧ�����������������������ڶ�������������������������ˮ����������ΪA�в���������������C�е�ʯ��ˮ����ǣ�֤���ж�����̼������ʯ��ˮ��Ӧ����������̼������B�������ȼ�գ����ݷ�Ӧǰ��Ԫ��������ԭ��֤�������к���̼Ԫ�أ��ʴ�Ϊ��˫��ˮ
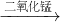 ˮ+�������ԣ���ɫ������CaCO3������̼Ԫ�أ�CΪCa��OH��2��Һ����̼Ԫ�أ�A�����ɵ�����Ϊ��������̼Ԫ�أ���ѧ��Ӧǰ��Ԫ������䣬����̼Ԫ������������
ˮ+�������ԣ���ɫ������CaCO3������̼Ԫ�أ�CΪCa��OH��2��Һ����̼Ԫ�أ�A�����ɵ�����Ϊ��������̼Ԫ�أ���ѧ��Ӧǰ��Ԫ������䣬����̼Ԫ��������������3����������ˮ�Ĺ����ˮ���룬���ù��˵ķ������ʴ�Ϊ������
��4��D�д�ŵ���C��������Һ������ʯ��ˮ��������еĶ�����̼��Ӧ����̼��ƣ���˰�Ĥ����Ҫ�ɷ���̼��ƣ��ʴ�Ϊ��̼��ƣ�
������ʵ������ȡCO2�����ڳ����£���̼��ƺ����ụ�ཻ���ɷ������Ȼ��ƺ�ˮ�Ͷ�����̼����˲���Ҫ���ȣ�������̼������ˮ���ܶȱȿ������ܶȴ����ֻ���������ſ������ռ�����1��B�е�����Ϩ��˵�����������岻֧��ȼ�գ��Ƕ�����̼���壻̼��ƺ����ᷴӦ�����Ȼ��ƺ�ˮ�Ͷ�����̼��C�е���Һ����ǣ�˵��������̼��ˮ��Ӧ����̼��ư�ɫ������ˮ����2����ʹ����ȼ�յø�����������������˵��A�з�Ӧ�����������������������ڶ�������������������������ˮ����������ΪA�в���������������C�е�ʯ��ˮ����ǣ�֤���ж�����̼������ʯ��ˮ��Ӧ����������̼������B�������ȼ�գ����ݷ�Ӧǰ��Ԫ��������ԭ��֤�������к���̼Ԫ�أ���3����������ˮ�Ĺ����ˮ���룬���ù��˵ķ�������4��D�д�ŵ���C��������Һ������ʯ��ˮ��������еĶ�����̼��Ӧ����̼��ƣ���˰�Ĥ����Ҫ�ɷ���̼��ƣ�
��������������Ҫ�����������ȼ�ա��������ȡװ�ú��ռ�װ�õ�ѡ��ͬʱҲ���������ֱ���ʽ����д���˵ȣ��ۺ��ԱȽ�ǿ���������ȡװ�õ�ѡ���뷴Ӧ���״̬�ͷ�Ӧ�������йأ�������ռ�װ�õ�ѡ����������ܶȺ��ܽ����йأ����������п�����Ҫ����֮һ����Ҫ������ʵ�����У�

С����С�������������װ����ʵ�飬A�����ӵ�ҩƷ��ͬ��C������ ҩƷ��ͬ��B�ж���һ��ȼ�ŵ�������ʱ�����ܳ���ȼ�ա�D��ʢ�ŵ���C��������Һ��
ҩƷ��ͬ��B�ж���һ��ȼ�ŵ�������ʱ�����ܳ���ȼ�ա�D��ʢ�ŵ���C��������Һ��
 | |||
 | |||
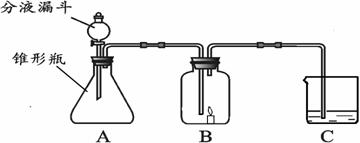
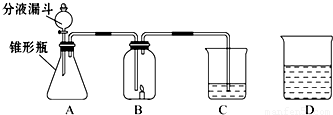
��1��С���ڷ�Һ©������ƿ��C�зֱ����ҩƷ����ʹ֮��ϣ��ٸ���B�����ӣ����ӳ���ͼ��ʾ������B�е�����Ϩ��C�е���Һ����ǣ�д��A�з�����Ӧ�ķ���ʽ___________
___________________��C�з�Ӧ�ķ���ʽ___________________________________��
��2��С��Ҳ�ڷ�Һ©������ƿ��C�зֱ����ҩƷ����ʹ֮��ϣ��ٸ���B�����ӣ����ӳ���ͼ��ʾ������B�е�����ȼ�յĸ�����C�е���ҺҲ����ǣ�д��A�з�����Ӧ�ķ���ʽ___________________________________��С��˵C�е�����֤�������к���̼Ԫ�أ�����ΪС��˵�Ķ���______________�� ���������___________________________________��
��3��С�����һ��̽��C�а�ɫ������ijɷ֣���C�еİ�ɫ�������ˮ��Һ�з�������ˣ����õķ�����___________________��
��4��С��Ҳ��̽�ְ�ɫ������ijɷ֣����õι�ȡC�еĻ���Һһ����һ�ɾ��IJ���Ƭ�ϣ�����һ��_____________����Һ����塣
��5���¿�ʱ����λͬѧ������û�����D��Һ������һ���Ĥ���ð�Ĥ�Ļ�ѧ�ɷֵ�������____________��
 С����С�������������װ����ʵ�飬A�����ӵ�ҩƷ��ͬ��C������ҩƷ��ͬ��B�ж���һ��ȼ�ŵ�������ʱ�����ܳ���ȼ�գ�D��ʢ�ŵ���C��������Һ��
С����С�������������װ����ʵ�飬A�����ӵ�ҩƷ��ͬ��C������ҩƷ��ͬ��B�ж���һ��ȼ�ŵ�������ʱ�����ܳ���ȼ�գ�D��ʢ�ŵ���C��������Һ��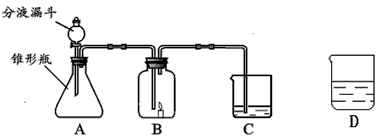
 С����С�������������װ����ʵ�飬A�����ӵ�ҩƷ��ͬ��C������ҩƷ��ͬ��B�ж���һ��ȼ�ŵ�������ʱ�����ܳ���ȼ�գ�D��ʢ�ŵ���C��������Һ��
С����С�������������װ����ʵ�飬A�����ӵ�ҩƷ��ͬ��C������ҩƷ��ͬ��B�ж���һ��ȼ�ŵ�������ʱ�����ܳ���ȼ�գ�D��ʢ�ŵ���C��������Һ��