��Ŀ����
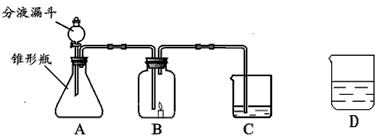
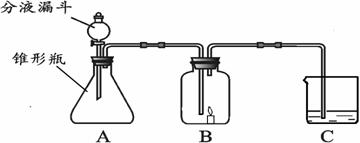
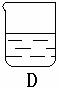
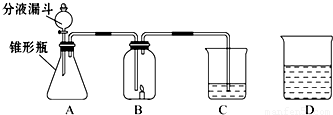 С����С�������������װ����ʵ�飬A�����ӵ�ҩƷ��ͬ��C������ҩƷ��ͬ��B�ж���һ��ȼ�ŵ�������ʱ�����ܳ���ȼ�գ�D��ʢ�ŵ���C��������Һ��
С����С�������������װ����ʵ�飬A�����ӵ�ҩƷ��ͬ��C������ҩƷ��ͬ��B�ж���һ��ȼ�ŵ�������ʱ�����ܳ���ȼ�գ�D��ʢ�ŵ���C��������Һ����1��С���ڷ�Һ©������ƿ��C�зֱ����ҩƷ����ʹ֮��ϣ��ٸ���B�����ӣ����ӳ���ͼ��ʾ������B�е�����Ϩ��C�е���Һ����ǣ�д��A�з�����Ӧ�ķ���ʽ______��C�з�Ӧ�ķ���ʽ______��
��2��С��Ҳ�ڷ�Һ©������ƿ��C�зֱ����ҩƷ����ʹ֮��ϣ��ٸ���B�����ӣ����ӳ���ͼ��ʾ������B�е�����ȼ�յĸ�����C�е���ҺҲ����ǣ�д��A�з�����Ӧ�ķ���ʽ______ 2H2O+O2��
��2��С����ʵ��ʱ����F�е�����ȼ�յĸ�����˵�������������������д��ѧ����ʽ�����������غ㶨�ɽ��з������
��3���������֪��G�а�ɫ����Ϊ̼��ƣ�����������ˮ�������ù��˵ķ�����
��4������̼��������ᷴӦ��
��5��ʯ��ˮ�ijɷ���Ca��OH��2����������е�CO2������Ӧ�����н��
����⣺��1��С����ʱ����F�е�����Ϩ��˵�����ɶ�����̼����A�з�����Ӧ�ķ���ʽ��CaCO3+2HCl=CaCl2+CO2��+H2O�� C�е���Һ����ǣ�C��ҺΪ����������Һ��C�з�Ӧ�ķ���ʽ Ca��OH��2+CO2=CaCO3��+H2O��
��2��С����ʵ��ʱ����F�е�����ȼ�յĸ�����˵��������������A�з�����Ӧ�ķ���ʽΪ��2H2O2
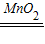 2H2O+O2����C�е���ҺҲ������ж�����̼���ɣ����������غ㶨��֤�������к���̼Ԫ�أ�����С��˵�Ķԣ�
2H2O+O2����C�е���ҺҲ������ж�����̼���ɣ����������غ㶨��֤�������к���̼Ԫ�أ�����С��˵�Ķԣ���3���������֪��G�а�ɫ����Ϊ̼��ƣ�����������ˮ�������ù��˵ķ�����
��4������̼��������ᷴӦ��
��5��ʯ��ˮ�ijɷ���Ca��OH��2����������е�CO2������Ӧ����ѧ����ʽʽΪ CO2+Ca��OH��2�TCaCO3��+H2O����˴˰�Ĥ��̼��ƣ�
�ʴ�Ϊ����1��CaCO3+2HCl=CaCl2+CO2��+H2O�� Ca��OH��2+CO2=CaCO3��+H2O��
��2��2H2O2
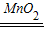 2H2O+O2�����ԣ�C�е���ҺҲ������ж�����̼���ɣ����������غ㶨��֤�������к���̼Ԫ�أ�
2H2O+O2�����ԣ�C�е���ҺҲ������ж�����̼���ɣ����������غ㶨��֤�������к���̼Ԫ�أ���3�����ˣ� ��4��HCl�� ��5��̼��ƣ�
���������������Ͷ�����̼�����ʺͼ��鷽����������Ŀ�е���Ϣ��ȷ��д��ѧ����ʽ���Լ���֪ʶӦ�õ������е�������������ѧΪ���õ�ԭ��

С����С�������������װ����ʵ�飬A�����ӵ�ҩƷ��ͬ��C������ ҩƷ��ͬ��B�ж���һ��ȼ�ŵ�������ʱ�����ܳ���ȼ�ա�D��ʢ�ŵ���C��������Һ��
ҩƷ��ͬ��B�ж���һ��ȼ�ŵ�������ʱ�����ܳ���ȼ�ա�D��ʢ�ŵ���C��������Һ��
 | |||
 | |||
��1��С���ڷ�Һ©������ƿ��C�зֱ����ҩƷ����ʹ֮��ϣ��ٸ���B�����ӣ����ӳ���ͼ��ʾ������B�е�����Ϩ��C�е���Һ����ǣ�д��A�з�����Ӧ�ķ���ʽ___________
___________________��C�з�Ӧ�ķ���ʽ___________________________________��
��2��С��Ҳ�ڷ�Һ©������ƿ��C�зֱ����ҩƷ����ʹ֮��ϣ��ٸ���B�����ӣ����ӳ���ͼ��ʾ������B�е�����ȼ�յĸ�����C�е���ҺҲ����ǣ�д��A�з�����Ӧ�ķ���ʽ___________________________________��С��˵C�е�����֤�������к���̼Ԫ�أ�����ΪС��˵�Ķ���______________�� ���������___________________________________��
��3��С�����һ��̽��C�а�ɫ������ijɷ֣���C�еİ�ɫ�������ˮ��Һ�з�������ˣ����õķ�����___________________��
��4��С��Ҳ��̽�ְ�ɫ������ijɷ֣����õι�ȡC�еĻ���Һһ����һ�ɾ��IJ���Ƭ�ϣ�����һ��_____________����Һ����塣
��5���¿�ʱ����λͬѧ������û�����D��Һ������һ���Ĥ���ð�Ĥ�Ļ�ѧ�ɷֵ�������____________��
 С����С�������������װ����ʵ�飬A�����ӵ�ҩƷ��ͬ��C������ҩƷ��ͬ��B�ж���һ��ȼ�ŵ�������ʱ�����ܳ���ȼ�գ�D��ʢ�ŵ���C��������Һ��
С����С�������������װ����ʵ�飬A�����ӵ�ҩƷ��ͬ��C������ҩƷ��ͬ��B�ж���һ��ȼ�ŵ�������ʱ�����ܳ���ȼ�գ�D��ʢ�ŵ���C��������Һ��