��Ŀ����
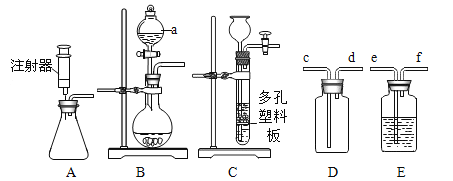
����Ŀ��ijͬѧ���������ͼ��ʾ��װ�ã�����ʵ������ȡCO2���������IJ������ʽ���̽������Ҫ��ش����⡣
(1)д�����������ƣ�m__________��n_____________��
(2)Aװ��������ȡCO2����Ӧ�Ļ�ѧ����ʽΪ _________���÷���װ�õ��ŵ���________________�����)
a�����Ʒ�Ӧ���� b�����Ʒ�Ӧ�ķ�����ֹͣ
(3)�� B װ���ռ� CO2������Ӧ��____(ѡ����a������b��)��ͨ�룬���� CO2 �Ƿ���ʱ������һ�˹ܿڷ�һ_______��ľ����
(4)��ҪC �е��������������ڿ����У������������__________(�����)��
A������ B������ C������
(5)�� D�Թ���ͨ��CO2��������________����ʾʯ��ˮ�е�����ǡ����ȫ������ ��д�� CO2��ʯ��ˮ��Ӧ�Ļ�ѧ����ʽ____________��
(6)��20mL��ע�����ȳ�ȡ10mlˮ���ٳ�ȡ10mLCO2(��ͼE)�������ܺ��������۲쵽�������ͣ��12ml�̶ȴ���˵����������10mlˮԼ���ܽ�_______mlCO2��
���𰸡���Һ©�� ��ƿ CaCO3+2HCl=CaCl2��H2O��CO2�� a a ȼ�� A ��ɫ�պ���ȥ CO2+Ca(OH)2=CaCO3��+H2O 8
��������
��1������m������Ϊ����Һ©��������n������Ϊ����ƿ��
��2��AΪ��Һ�������ͷ�Ӧװ�ã�̼��ƺ�ϡ���ᷴӦ��ȡ������̼���ô�װ�ã���Ӧ�ķ���ʽΪ��CaCO3+2HCl=CaCl2��H2O��CO2�����÷���װ�ÿɿ��Ʒ�Ӧ���ʣ����ܿ��Ʒ�Ӧ�ķ�����ֹͣ����ѡa��
��3��CO2���ܶȱȿ�������Bװ���ռ� CO2������Ӧ��b���ݳ���������Ӧ��a��ͨ�룬���� CO2 �Ƿ���ʱ������һ�˹ܿڷ�һȼ�ŵ�ľ������ľ��Ϩ�������弯����
��4����ҪC �е��������������ڿ����У���������ܶ�ӦС�ڿ������������������
��5��Ca(OH)2�ʼ��ԣ���̪�������б�죬��D�Թ���ͨ��CO2��CO2��Ca(OH)2��Ӧ�������ֺ�ɫ�պ���ȥ����ʾʯ��ˮ�е�����ǡ����ȫ������CO2��ʯ��ˮ��Ӧ�Ļ�ѧ����ʽCO2+Ca(OH)2=CaCO3��+H2O��
��6����20mL��ע�����ȳ�ȡ10mlˮ���ٳ�ȡ10mLCO2�������ܺ��������۲쵽�������ͣ��12ml�̶ȴ���˵����������10mlˮԼ���ܽ�8mlCO2��

����Ŀ����������Ҫ�Ľ������ϣ��ڹ�ũҵ������������Ӧ�÷dz��㷺��
I������Ӧ��
��1���߿Ƽ���Ʒ��������-Fe������������ʳƷ���ʣ���֮Ϊ��˫����������Ϊ�������տ����е�_________��
II������ұ��
��ҵ������ԭ���Ǹ�������CO����ԭ�����������������л�ԭ��������ش��������⣺
��1��д���Գ�����Ϊԭ�ϣ��ڸ����������Ļ�ѧ����ʽ��__________________��
��2����¯�����У���̿�����ó��˿�������һ����̼�⣬����______________��
�����Ļ��̽��
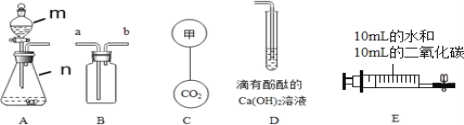
����һ������AgNO3��Cu (NO3)2�����Һ����������ͼ��ʾ��ʵ�飬������ҺA����B�ijɷֽ����˷������о���
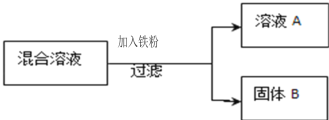
��������⣩��ҺA�е����ʿ�������Щ��
���������룩
��ֻ��Fe(NO3)2
����Fe (NO3)2��Cu (NO3)2
�� ��Fe (NO3)2��AgNO3
�� ��Fe (NO3)2��Cu (NO3)2��AgNO3
���������ۣ��������IJ�����__�����ţ�����������_______
��ʵ��̽������������е�ʵ�鲽�輰������ɱ��е�ʵ�����
ʵ�鲽�� | ���� | ʵ����� |
ȡ��������B���μ� ϡ���� | �����ݲ��� | ��ҺA�е����ʳɷַ��� ����_____������B�еĽ����ɷ���________�� |
�����������������IJⶨ
ij������ȤС��ⶨ�����������ʵ��������ʼȲ�����ˮҲ�������ᣩ��������������������ȡ�����Ʒ���ֱ��ϡ���ᷴӦ����ò����������£�������й���Ϣ�ش����⣮
ʵ����� | 1 | 2 | 3 | 4 | 5 |
ȡ��Ʒ������g�� | 30.0 | 30.0 | 30.0 | 30.0 | 30.0 |
ȡϡ����������g�� | 50.0 | 100.0 | 150.0 | 200.0 | 250.0 |
��������������g��/span> | 0.2 | a | 0.6 | 0.8 | 0.9 |
��1��a����ֵΪ______��
��2����ʽ�������Ʒ�е���������������______��
��3����5��ʵ����������õ���Һ�����ʵĻ�ѧʽΪ_______��
��4����1��ʵ����������õ���Һ��������������______����ʽ����д���̡�