��Ŀ����
����Ŀ����Դ����ʯ�ͻ�����ս��Ŀ�꣬��չ��CH4 ��CO2��Ϊԭ�ϵġ�C1��ѧ����Ϊ���������ı�Ȼ���ơ�ͨ����Ȼ���к���H2S���ж����壬��ͼΪ��Ȼ���ϳɰ��Ĺ������̡�

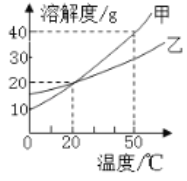
(1)����----����ȼ�ϵ���ǽ�______��ת��Ϊ______�ܵ�װ�á�CH4�ڿ�����ȼ��ʱ���������С�ձ����ڻ����Ϸ����ɹ۲쵽�ձ��ڱ���______��
(2)CH4�ɺϳɶ���������л���о�CH4��CO2��Ӧ����CO��H2���ش���������________���ҹ�������Ա���ȷ���CH4��ֱ�ӷֽ���C6H6��H2����ѧ����ʽΪ___________��
(3)����CH4��H2O(������Ӧ����CO2��H2����ѧ����ʽ��______������һ�����K2CO3��Һ����CO2���˷�Ӧ��ʾΪK2CO3��CO2��H2O = 2_________��N2 �� H2���շ�����1��____��Ӧ����NH3��
���𰸡� ��ѧ �� ��ɫҺ�� ��������ʯ�ͻ�����Դ 6CH4=C6H6+9H2 CH4+2H2O(��)=CO2+4H2 KHCO3 3
��������������ѧ֪ʶ��������Ϣ֪��(1)����----����ȼ�ϵ���ǽ���ѧ��ת��Ϊ���ܵ�װ�á�CH4�ڿ�����ȼ��ʱ���������С�ձ����ڻ����Ϸ����ɹ۲쵽�ձ��ڱ�����ɫҺ�Ρ�
(2 )CH4�ɺϳɶ���������л���о�CH4��CO2��Ӧ����CO��H2���ش��������ڿ�������ʯ�ͻ�����Դ ���ҹ�������Ա���ȷ���CH4��ֱ�ӷֽ���C6H6��H2����ѧ����ʽΪ6CH4=C6H6+9H2��(3)����CH4��H2O(������Ӧ����CO2��H2����ѧ����ʽ��CH4+2H2O(��)=CO2+4H2������һ�����K2CO3��Һ����CO2���˷�Ӧ��ʾΪK2CO3��CO2��H2O = 2 KHCO3��N2��3H2��2NH3 �� N2 �� H2���շ�����1��3��Ӧ����NH3��
�㾦��������Ҫ�������ʵ����ʡ�

 Сѧ��10���ӿ������100��ϵ�д�
Сѧ��10���ӿ������100��ϵ�д�