��Ŀ����
С����ѧϰ�˴��Na2CO3�������ʺ����뵽����������ͷʱ���õ�����С�մ�NaHCO3��,���Ǵӳ���ȡ����С�մ���Ʒ����������ʵ��̽����
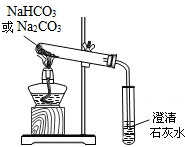
ʵ��һ��̽��̼�����ƵĻ�ѧ����
��1��ȡ��Ʒ����������ˮ�У������Һ��pH����7�����������ж�NaHCO3��Һ�ԣ������ԡ����ԡ����ԡ���______�ԡ�
��2����С�մ���Ʒ�еμ����ᣬ�д������ݲ�����������������Ƕ�����̼���÷�Ӧ�Ļ�ѧ����ʽΪ________________________��
��3�������ɵ�CO2�л���������SO2Ϊ�˳�ȥSO2��������������ַ�����
������ ������ͨ��������̼��������Һ��
�ҷ�����������ͨ������������������Һ��
[�ṩ����]����. CO2��NaHCO3���ᷢ����Ӧ��
��.SO2+2 NaHCO3==Na2SO3+H2O+2CO2
��.SO2+2NaOH==Na2SO4+H2O
���������ṩ�����Ϻ���ѧ��֪ʶ����С������������_______��������ס����ҡ������У�������_______________��
ʵ�����̽��̼���ƺ�̼�����Ƶ����ȶ���
(4)С��ͨ���������ϣ�����ʦ��ָ���²�����ͼ��װ�öԴ����С�մ����˷ֱ���ȵĶԱ�ʵ�飨����ʱ�䡢�����¶���ͬ�������ȴ���ʱ�Թ��еij���ʯ��ˮ������������С�մ�ʱ�Թ��еij���ʯ��ˮ���ְ�ɫ���ǣ��Իش�
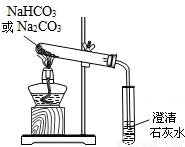
ʵ��һ��̽��̼�����ƵĻ�ѧ����
��1��ȡ��Ʒ����������ˮ�У������Һ��pH����7�����������ж�NaHCO3��Һ�ԣ������ԡ����ԡ����ԡ���______�ԡ�
��2����С�մ���Ʒ�еμ����ᣬ�д������ݲ�����������������Ƕ�����̼���÷�Ӧ�Ļ�ѧ����ʽΪ________________________��
��3�������ɵ�CO2�л���������SO2Ϊ�˳�ȥSO2��������������ַ�����
������ ������ͨ��������̼��������Һ��
�ҷ�����������ͨ������������������Һ��
[�ṩ����]����. CO2��NaHCO3���ᷢ����Ӧ��
��.SO2+2 NaHCO3==Na2SO3+H2O+2CO2
��.SO2+2NaOH==Na2SO4+H2O
���������ṩ�����Ϻ���ѧ��֪ʶ����С������������_______��������ס����ҡ������У�������_______________��
ʵ�����̽��̼���ƺ�̼�����Ƶ����ȶ���
(4)С��ͨ���������ϣ�����ʦ��ָ���²�����ͼ��װ�öԴ����С�մ����˷ֱ���ȵĶԱ�ʵ�飨����ʱ�䡢�����¶���ͬ�������ȴ���ʱ�Թ��еij���ʯ��ˮ������������С�մ�ʱ�Թ��еij���ʯ��ˮ���ְ�ɫ���ǣ��Իش�

�ټ���С�մ�Ļ�ѧ��Ӧ����ʽ��_____________________
��ʵ��ó��Ľ�����___________________________��
��ʵ��ó��Ľ�����___________________________��
��1����
��2��NaHCO3��HCl==NaCl+CO2����H2O
��3���ף�CO2��NaHCO3���ᷢ����Ӧ��SO2��NaHCO3�ܷ�Ӧ�������ܳ�ȥSO2�����һ�������CO2�ĺ���
��4����2NaHCO3 Na2CO3+CO2����H2O
Na2CO3+CO2����H2O
��̼���������Ȼ�ֽ⣬̼�������Ȳ��ֽ⣨��̼���Ʊ�̼�������ȶ���
��2��NaHCO3��HCl==NaCl+CO2����H2O
��3���ף�CO2��NaHCO3���ᷢ����Ӧ��SO2��NaHCO3�ܷ�Ӧ�������ܳ�ȥSO2�����һ�������CO2�ĺ���
��4����2NaHCO3
 Na2CO3+CO2����H2O
Na2CO3+CO2����H2O��̼���������Ȼ�ֽ⣬̼�������Ȳ��ֽ⣨��̼���Ʊ�̼�������ȶ���

��ϰ��ϵ�д�
 ������ĩ��ϰ��ѵ��ϵ�д�
������ĩ��ϰ��ѵ��ϵ�д� С��ʿ��ĩ����100��ϵ�д�
С��ʿ��ĩ����100��ϵ�д�
�����Ŀ
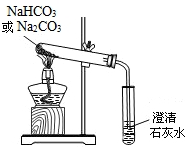 С����ѧϰ�˴��Na2CO3�������ʺ����뵽����������ͷʱ���õ�����С�մ�NaHCO3�������Ǵӳ���ȡ����С�մ���Ʒ����������ʵ��̽����
С����ѧϰ�˴��Na2CO3�������ʺ����뵽����������ͷʱ���õ�����С�մ�NaHCO3�������Ǵӳ���ȡ����С�մ���Ʒ����������ʵ��̽���� С����ѧϰ�˴��Na2CO3�������ʺ����뵽����������ͷʱ���õ�����С�մ�NaHCO3�������Ǵӳ���ȡ����С�մ���Ʒ����������ʵ��̽����
С����ѧϰ�˴��Na2CO3�������ʺ����뵽����������ͷʱ���õ�����С�մ�NaHCO3�������Ǵӳ���ȡ����С�մ���Ʒ����������ʵ��̽����