��Ŀ����
ʵ������ȡ�����ǻ�ѧѧϰ�Ļ���ʵ�鼼��֮һ���������ͼ�ش��������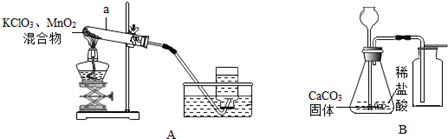
��1�������a�������� ����
��2����ͬѧ��Aװ����ȡ�������÷�Ӧ�Ļ�ѧ����ʽ�� �����÷�Ӧ�Ļ�����Ӧ�������� ����ֹͣ����ʱӦ�Ƚ������Ƴ�ˮ�棬���������� ����
��3�����鼯��ƿ�е������������ķ���Ϊ��
��4����ͬѧ��Bװ����ȡ������̼���÷�Ӧ�Ļ�ѧ����ʽΪ�� �����ռ����Ķ�����̼�п��ܺ��е��������� ����Ҫ��ȥ��Щ�����õ����Լ��Ⱥ����� ����
��1���Թܣ���2��2KClO3 2KCl+3O2�����ֽⷴӦ����ֹˮ���������Թ�ը�ѣ���3���������ǵ�ľ�����뼯��ƿ�ڣ�ľ����ȼ˵������ƿ�е���������������4��CaCO3+2HCl�TCaCl2+H2O+CO2�����Ȼ����ˮ���������͵�̼��������Һ��Ũ���ᣮ
2KCl+3O2�����ֽⷴӦ����ֹˮ���������Թ�ը�ѣ���3���������ǵ�ľ�����뼯��ƿ�ڣ�ľ����ȼ˵������ƿ�е���������������4��CaCO3+2HCl�TCaCl2+H2O+CO2�����Ȼ����ˮ���������͵�̼��������Һ��Ũ���ᣮ
����

��ϰ��ϵ�д�
�����Ŀ