��Ŀ����
ij���������ð�������Ĵ����Ʒ�к��������Ȼ������ʣ����Ʒ��װ���ϱ�����̼���ơ�96%��Ϊ�ⶨ�ò�Ʒ�к�̼���Ƶ���������������������ʵ�飻ȡ11.0g������Ʒ�����ձ��У��Ƶ��ձ�����ʢ������Ʒ��������Ϊ158.0g���ٰ�100gϡ����ƽ���ֳ��ķ����μ�����Ʒ�У���ַ�Ӧ��ʵ�����ݼ�¼���£�
����ݴ˷������㣺
��1����һ�μ���ϡ�����ַ�Ӧ�����ɶ�����̼��������______g��
��2���ò�Ʒ��̼���Ƶ�����������______���������ȷ��0.1%��
| ��������Ĵ��� | ��һ�� | �ڶ��� | ������ | ���Ĵ� |
| �ձ�����ʢ���ʵ�������/g | 181.2 | 204.4 | 228.6 | 253.6 |
��1����һ�μ���ϡ�����ַ�Ӧ�����ɶ�����̼��������______g��
��2���ò�Ʒ��̼���Ƶ�����������______���������ȷ��0.1%��
�ɱ������ݿ�֪����һ�����ɶ�����̼������Ϊ��158.0g+100g��4-181.2g=1.8g
��ַ�Ӧ�����ɶ�����̼������Ϊ��158.0g+100g-253.6g=4.4g
����Ʒ�к��е�̼���Ƶ�����ΪX
Na2CO3+2HCl=2NaCl+H2O+CO2��
106 44
X 4.4g
=
X=10.6g
̼���Ƶ���������Ϊ��
��100%=96.4%
�ʴ�Ϊ��
��1��1.8
��2��96.4%
��ַ�Ӧ�����ɶ�����̼������Ϊ��158.0g+100g-253.6g=4.4g
����Ʒ�к��е�̼���Ƶ�����ΪX
Na2CO3+2HCl=2NaCl+H2O+CO2��
106 44
X 4.4g
| 106 |
| X |
| 44 |
| 4.4g |
X=10.6g
̼���Ƶ���������Ϊ��
| 10.6g |
| 11.0g |
�ʴ�Ϊ��
��1��1.8
��2��96.4%

��ϰ��ϵ�д�
�����Ŀ
��1��̼�����Dz�������ֽ��������ϴ�Ӽ�����֯���Ƹ�ȹ�ҵ����Ҫԭ�ϣ�������
����ᡱ��������Ρ��������������д����ԭ���� ��
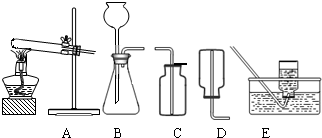
��2����ˮ̼���ƵĹ�ҵ�Ʒ���Ҫ�а���������Ƽ���֣��������ʳ�Σ��Ȼ��ƣ���ʯ��ʯ��������������ʯ�ҺͶ�����̼������������������ˮ��Ϊԭ������ȡ�����ҵ������N2��H2�ڸ��¡���ѹ�ʹ��������ºϳɰ��ģ�ʵ����һ�����Ȼ�狀���ʯ�ҹ������Ʊ������İ������÷�Ӧ�Ļ�ѧ����ʽΪ ����ȡ���ռ�����������ͼ�е� �� װ�ã�
��3��ij���������ð�������Ĵ����Ʒ�к��������Ȼ������ʣ����Ʒ��װ����ע����̼���ơ�96%��Ϊ�ⶨ�ò�Ʒ�к�̼���Ƶ���������������������ʵ�飬ȡ11.0g������Ʒ�����ձ��У��Ƶ��ձ�����ʢ������Ʒ��������Ϊ158.0g���ٰ�100gϡ����ƽ���ֳ��ķ����μ�����Ʒ�У�ÿ�ξ���ַ�Ӧ��ʵ�����ݼ�¼���£�
����ݴ˷������㣺
�ٵ�һ�μ���ϡ�����ַ�Ӧ�����ɶ�����̼�������� g��
�ڸò�Ʒ��̼���Ƶ����������Ƿ�ϸ�Ҫ��д��������̣������ȷ��0.1%��
��2����ˮ̼���ƵĹ�ҵ�Ʒ���Ҫ�а���������Ƽ���֣��������ʳ�Σ��Ȼ��ƣ���ʯ��ʯ��������������ʯ�ҺͶ�����̼������������������ˮ��Ϊԭ������ȡ�����ҵ������N2��H2�ڸ��¡���ѹ�ʹ��������ºϳɰ��ģ�ʵ����һ�����Ȼ�狀���ʯ�ҹ������Ʊ������İ������÷�Ӧ�Ļ�ѧ����ʽΪ

��3��ij���������ð�������Ĵ����Ʒ�к��������Ȼ������ʣ����Ʒ��װ����ע����̼���ơ�96%��Ϊ�ⶨ�ò�Ʒ�к�̼���Ƶ���������������������ʵ�飬ȡ11.0g������Ʒ�����ձ��У��Ƶ��ձ�����ʢ������Ʒ��������Ϊ158.0g���ٰ�100gϡ����ƽ���ֳ��ķ����μ�����Ʒ�У�ÿ�ξ���ַ�Ӧ��ʵ�����ݼ�¼���£�
| ��������Ĵ��� | ��һ�� | �ڶ��� | ������ | ���Ĵ� |
| �ձ�����ʢ���ʵ�������/g | 181.2 | 204.4 | 228.6 | 253.6 |
�ٵ�һ�μ���ϡ�����ַ�Ӧ�����ɶ�����̼��������
�ڸò�Ʒ��̼���Ƶ����������Ƿ�ϸ�Ҫ��д��������̣������ȷ��0.1%��
��1��̼�����Dz�������ֽ��������ϴ�Ӽ�����֯���Ƹ�ȹ�ҵ����Ҫԭ�ϣ�������
______����ᡱ��������Ρ��������������д����ԭ����______��
��2����ˮ̼���ƵĹ�ҵ�Ʒ���Ҫ�а���������Ƽ���֣��������ʳ�Σ��Ȼ��ƣ���ʯ��ʯ��������������ʯ�ҺͶ�����̼������������������ˮ��Ϊԭ������ȡ�����ҵ������N2��H2�ڸ��¡���ѹ�ʹ��������ºϳɰ��ģ�ʵ����һ�����Ȼ�狀���ʯ�ҹ������Ʊ������İ������÷�Ӧ�Ļ�ѧ����ʽΪ______����ȡ���ռ�����������ͼ�е�______��______װ�ã�

��3��ij���������ð�������Ĵ����Ʒ�к��������Ȼ������ʣ����Ʒ��װ����ע����̼���ơ�96%��Ϊ�ⶨ�ò�Ʒ�к�̼���Ƶ���������������������ʵ�飬ȡ11.0g������Ʒ�����ձ��У��Ƶ��ձ�����ʢ������Ʒ��������Ϊ158.0g���ٰ�100gϡ����ƽ���ֳ��ķ����μ�����Ʒ�У�ÿ�ξ���ַ�Ӧ��ʵ�����ݼ�¼���£�
| ��������Ĵ��� | ��һ�� | �ڶ��� | ������ | ���Ĵ� |
| �ձ�����ʢ���ʵ�������/g | 181.2 | 204.4 | 228.6 | 253.6 |
�ٵ�һ�μ���ϡ�����ַ�Ӧ�����ɶ�����̼��������______g��
�ڸò�Ʒ��̼���Ƶ����������Ƿ�ϸ�Ҫ��д��������̣������ȷ��0.1%��
��1��̼�����Dz�������ֽ��������ϴ�Ӽ�����֯���Ƹ�ȹ�ҵ����Ҫԭ�ϣ�������
______����ᡱ��������Ρ��������������д����ԭ����______��
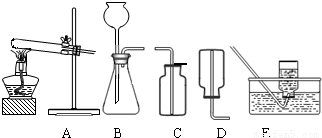
��2����ˮ̼���ƵĹ�ҵ�Ʒ���Ҫ�а���������Ƽ���֣��������ʳ�Σ��Ȼ��ƣ���ʯ��ʯ��������������ʯ�ҺͶ�����̼������������������ˮ��Ϊԭ������ȡ�����ҵ������N2��H2�ڸ��¡���ѹ�ʹ��������ºϳɰ��ģ�ʵ����һ�����Ȼ�狀���ʯ�ҹ������Ʊ������İ������÷�Ӧ�Ļ�ѧ����ʽΪ______����ȡ���ռ�����������ͼ�е�______��______װ�ã�
��3��ij���������ð�������Ĵ����Ʒ�к��������Ȼ������ʣ����Ʒ��װ����ע����̼���ơ�96%��Ϊ�ⶨ�ò�Ʒ�к�̼���Ƶ���������������������ʵ�飬ȡ11.0g������Ʒ�����ձ��У��Ƶ��ձ�����ʢ������Ʒ��������Ϊ158.0g���ٰ�100gϡ����ƽ���ֳ��ķ����μ�����Ʒ�У�ÿ�ξ���ַ�Ӧ��ʵ�����ݼ�¼���£�
����ݴ˷������㣺
�ٵ�һ�μ���ϡ�����ַ�Ӧ�����ɶ�����̼��������______g��
�ڸò�Ʒ��̼���Ƶ����������Ƿ�ϸ�Ҫ��д��������̣������ȷ��0.1%��
______����ᡱ��������Ρ��������������д����ԭ����______��
��2����ˮ̼���ƵĹ�ҵ�Ʒ���Ҫ�а���������Ƽ���֣��������ʳ�Σ��Ȼ��ƣ���ʯ��ʯ��������������ʯ�ҺͶ�����̼������������������ˮ��Ϊԭ������ȡ�����ҵ������N2��H2�ڸ��¡���ѹ�ʹ��������ºϳɰ��ģ�ʵ����һ�����Ȼ�狀���ʯ�ҹ������Ʊ������İ������÷�Ӧ�Ļ�ѧ����ʽΪ______����ȡ���ռ�����������ͼ�е�______��______װ�ã�

��3��ij���������ð�������Ĵ����Ʒ�к��������Ȼ������ʣ����Ʒ��װ����ע����̼���ơ�96%��Ϊ�ⶨ�ò�Ʒ�к�̼���Ƶ���������������������ʵ�飬ȡ11.0g������Ʒ�����ձ��У��Ƶ��ձ�����ʢ������Ʒ��������Ϊ158.0g���ٰ�100gϡ����ƽ���ֳ��ķ����μ�����Ʒ�У�ÿ�ξ���ַ�Ӧ��ʵ�����ݼ�¼���£�
| ��������Ĵ��� | ��һ�� | �ڶ��� | ������ | ���Ĵ� |
| �ձ�����ʢ���ʵ�������/g | 181.2 | 204.4 | 228.6 | 253.6 |
�ٵ�һ�μ���ϡ�����ַ�Ӧ�����ɶ�����̼��������______g��
�ڸò�Ʒ��̼���Ƶ����������Ƿ�ϸ�Ҫ��д��������̣������ȷ��0.1%��
��1��̼�����Dz�������ֽ��������ϴ�Ӽ�����֯���Ƹ�ȹ�ҵ����Ҫԭ�ϣ�������
______����ᡱ��������Ρ��������������д����ԭ����______��
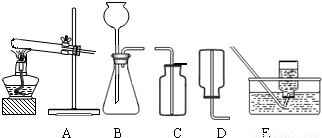
��2����ˮ̼���ƵĹ�ҵ�Ʒ���Ҫ�а���������Ƽ���֣��������ʳ�Σ��Ȼ��ƣ���ʯ��ʯ��������������ʯ�ҺͶ�����̼������������������ˮ��Ϊԭ������ȡ�����ҵ������N2��H2�ڸ��¡���ѹ�ʹ��������ºϳɰ��ģ�ʵ����һ�����Ȼ�狀���ʯ�ҹ������Ʊ������İ������÷�Ӧ�Ļ�ѧ����ʽΪ______����ȡ���ռ�����������ͼ�е�______��______װ�ã�
��3��ij���������ð�������Ĵ����Ʒ�к��������Ȼ������ʣ����Ʒ��װ����ע����̼���ơ�96%��Ϊ�ⶨ�ò�Ʒ�к�̼���Ƶ���������������������ʵ�飬ȡ11.0g������Ʒ�����ձ��У��Ƶ��ձ�����ʢ������Ʒ��������Ϊ158.0g���ٰ�100gϡ����ƽ���ֳ��ķ����μ�����Ʒ�У�ÿ�ξ���ַ�Ӧ��ʵ�����ݼ�¼���£�
����ݴ˷������㣺
�ٵ�һ�μ���ϡ�����ַ�Ӧ�����ɶ�����̼��������______g��
�ڸò�Ʒ��̼���Ƶ����������Ƿ�ϸ�Ҫ��д��������̣������ȷ��0.1%��
______����ᡱ��������Ρ��������������д����ԭ����______��
��2����ˮ̼���ƵĹ�ҵ�Ʒ���Ҫ�а���������Ƽ���֣��������ʳ�Σ��Ȼ��ƣ���ʯ��ʯ��������������ʯ�ҺͶ�����̼������������������ˮ��Ϊԭ������ȡ�����ҵ������N2��H2�ڸ��¡���ѹ�ʹ��������ºϳɰ��ģ�ʵ����һ�����Ȼ�狀���ʯ�ҹ������Ʊ������İ������÷�Ӧ�Ļ�ѧ����ʽΪ______����ȡ���ռ�����������ͼ�е�______��______װ�ã�

��3��ij���������ð�������Ĵ����Ʒ�к��������Ȼ������ʣ����Ʒ��װ����ע����̼���ơ�96%��Ϊ�ⶨ�ò�Ʒ�к�̼���Ƶ���������������������ʵ�飬ȡ11.0g������Ʒ�����ձ��У��Ƶ��ձ�����ʢ������Ʒ��������Ϊ158.0g���ٰ�100gϡ����ƽ���ֳ��ķ����μ�����Ʒ�У�ÿ�ξ���ַ�Ӧ��ʵ�����ݼ�¼���£�
| ��������Ĵ��� | ��һ�� | �ڶ��� | ������ | ���Ĵ� |
| �ձ�����ʢ���ʵ�������/g | 181.2 | 204.4 | 228.6 | 253.6 |
�ٵ�һ�μ���ϡ�����ַ�Ӧ�����ɶ�����̼��������______g��
�ڸò�Ʒ��̼���Ƶ����������Ƿ�ϸ�Ҫ��д��������̣������ȷ��0.1%��
��1��̼�����Dz�������ֽ��������ϴ�Ӽ�����֯���Ƹ�ȹ�ҵ����Ҫԭ�ϣ�������
______����ᡱ��������Ρ��������������д����ԭ����______��
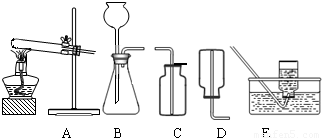
��2����ˮ̼���ƵĹ�ҵ�Ʒ���Ҫ�а���������Ƽ���֣��������ʳ�Σ��Ȼ��ƣ���ʯ��ʯ��������������ʯ�ҺͶ�����̼������������������ˮ��Ϊԭ������ȡ�����ҵ������N2��H2�ڸ��¡���ѹ�ʹ��������ºϳɰ��ģ�ʵ����һ�����Ȼ�狀���ʯ�ҹ������Ʊ������İ������÷�Ӧ�Ļ�ѧ����ʽΪ______����ȡ���ռ�����������ͼ�е�______��______װ�ã�
��3��ij���������ð�������Ĵ����Ʒ�к��������Ȼ������ʣ����Ʒ��װ����ע����̼���ơ�96%��Ϊ�ⶨ�ò�Ʒ�к�̼���Ƶ���������������������ʵ�飬ȡ11.0g������Ʒ�����ձ��У��Ƶ��ձ�����ʢ������Ʒ��������Ϊ158.0g���ٰ�100gϡ����ƽ���ֳ��ķ����μ�����Ʒ�У�ÿ�ξ���ַ�Ӧ��ʵ�����ݼ�¼���£�
����ݴ˷������㣺
�ٵ�һ�μ���ϡ�����ַ�Ӧ�����ɶ�����̼��������______g��
�ڸò�Ʒ��̼���Ƶ����������Ƿ�ϸ�Ҫ��д��������̣������ȷ��0.1%��
______����ᡱ��������Ρ��������������д����ԭ����______��
��2����ˮ̼���ƵĹ�ҵ�Ʒ���Ҫ�а���������Ƽ���֣��������ʳ�Σ��Ȼ��ƣ���ʯ��ʯ��������������ʯ�ҺͶ�����̼������������������ˮ��Ϊԭ������ȡ�����ҵ������N2��H2�ڸ��¡���ѹ�ʹ��������ºϳɰ��ģ�ʵ����һ�����Ȼ�狀���ʯ�ҹ������Ʊ������İ������÷�Ӧ�Ļ�ѧ����ʽΪ______����ȡ���ռ�����������ͼ�е�______��______װ�ã�

��3��ij���������ð�������Ĵ����Ʒ�к��������Ȼ������ʣ����Ʒ��װ����ע����̼���ơ�96%��Ϊ�ⶨ�ò�Ʒ�к�̼���Ƶ���������������������ʵ�飬ȡ11.0g������Ʒ�����ձ��У��Ƶ��ձ�����ʢ������Ʒ��������Ϊ158.0g���ٰ�100gϡ����ƽ���ֳ��ķ����μ�����Ʒ�У�ÿ�ξ���ַ�Ӧ��ʵ�����ݼ�¼���£�
| ��������Ĵ��� | ��һ�� | �ڶ��� | ������ | ���Ĵ� |
| �ձ�����ʢ���ʵ�������/g | 181.2 | 204.4 | 228.6 | 253.6 |
�ٵ�һ�μ���ϡ�����ַ�Ӧ�����ɶ�����̼��������______g��
�ڸò�Ʒ��̼���Ƶ����������Ƿ�ϸ�Ҫ��д��������̣������ȷ��0.1%��