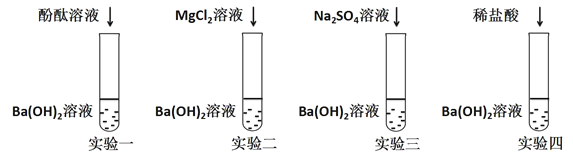
��Ŀ����
����Ŀ�������dz��л�ѧ������ʵ�顣��ش��������⣺
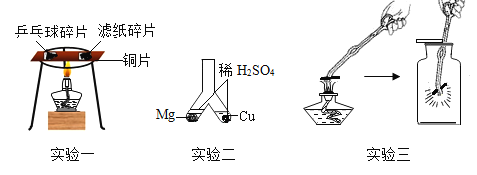
��1��ʵ��һ�й۲쵽��������_____________��ȼ�ţ��ƾ��Ƽ��ȱ�ͭƬ�м䲿λ��Ŀ����__________����ʵ��̽����ȼ�յ�������____________________________��
��2��ʵ����ܹ�֤��þ�Ļ�Դ���ͭ�Ļ�Ե�������_________________________��д��ʵ����з����Ļ�ѧ��Ӧ�Ļ�ѧ����ʽ___________________________��
��3��ʵ������ľ̿��������ȼ�յ�ʵ�飬ʵ�����֤ȼ�ղ���ʱ��ΪʲôҪ�ȼ���ƿ�¶���ȴ�����º��������е�����ʯ��ˮ��____________________________��
���𰸡�ƹ������Ƭ ʹƹ����Ƭ�ͺ���ֽƬ�������¶���ͬ �¶���Ҫ�ﵽ��ȼ����Ż�� ����Թ������ݲ������ұ�û������ Mg��H2SO4=MgSO4��H2�� �������Ƶ��ܽ�����¶ȵ����߶���С�����¶�û����ȴ�����£�����Ϊ�¶Ƚϸ��������ƴ���Һ��������Ӱ�����ļ���
��������
��1��ʵ��һ�й۲쵽��������ƹ������ȼ�ţ���Ϊƹ������Ż���¶ȵͣ��ƾ��Ƽ��ȱ�ͭƬ�м䲿λ��Ŀ���ǣ�ʹƹ�Ҿͺ���ֽƬ�������¶���ͬ����ʵ��̽����ȼ�յ������ǣ�ȼ��ʱ���¶���Ҫ�ﵽ��ȼ����Ż�㣻
��2��ʵ����ܹ�֤��þ�Ļ�Դ���ͭ�Ļ�Ե������ǣ�����Թ������ݲ������ұ�û�����ݣ���Ϊþ����ϡ���ᷴӦ������������ͭ���ܣ�ʵ�����þ��ϡ���ᷴӦ��������þ�������Ļ�ѧ����ʽ��Mg��H2SO4=MgSO4��H2����
��3��ʵ������ľ̿��������ȼ�յ�ʵ���в���������̼���壬ʵ�����֤ȼ�ղ���ʱ��Ҫ�ȼ���ƿ�¶���ȴ�����º��������е�����ʯ��ˮ��ԭ���������Ƶ��ܽ�����¶ȵ����߶���С�����¶�û����ȴ�����£�����Ϊ�¶Ƚϸ��������ƴ���Һ��������Ӱ�����ļ��顣

 ����������ϵ�д�
����������ϵ�д� �Ż���ҵ�Ϻ��Ƽ����׳�����ϵ�д�
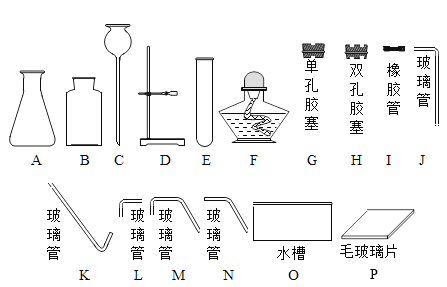
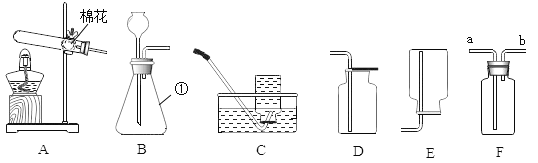
�Ż���ҵ�Ϻ��Ƽ����׳�����ϵ�д�����Ŀ����ʵ���ҽ�����ȡ������̼���塣�ɹ�ѡ���ʵ��װ�����¡�

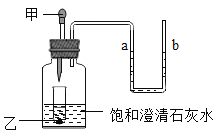
������װ���о������շ���������ԭ������_______ �����ţ�������a��������________��װ���ж���岻��ѡ����Ƭ���ʵ�ԭ����________ ���û�ѧ����ʽ��ʾ����
������ѡ������ȷװ�ã���������ʵ��̽����
ʵ��Ŀ��:______��
ʵ�鷽��:
ʵ���� | I | II | III |
ʯ��ʯ | 2.5g,��״ | 2.5g����ĩ״ | ______ |
���ᣨ������ | amL, 10%���� | amL, 20%���� | amL, 10%���� |
����ʵ�鷽�������Ʋ�ʵ��������Ҫ��_________�����ʵ�飬�۲�һ��ʱ���ڲ������ݵĿ�����
����ʵ�������õ�ʯ��ʯ��80%��CaCO3,�������ɶ�����̼_____________mol. �����ݻ�ѧ����ʽ��ʽ���㣩