��Ŀ����
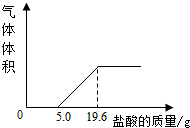
 СϣΪ�˲ⶨ���������������CO2������������������ʵ�飺���ռ�5L������������Ʒ������������Ʒ�м��������NaOH��Һ����ַ�Ӧ���۰ѷ�Ӧ�����Һת�Ƶ��ձ��У���������5%�����ᣬ������������Ϊֹ����������������������������Ĺ�ϵ��ͼ��
СϣΪ�˲ⶨ���������������CO2������������������ʵ�飺���ռ�5L������������Ʒ������������Ʒ�м��������NaOH��Һ����ַ�Ӧ���۰ѷ�Ӧ�����Һת�Ƶ��ձ��У���������5%�����ᣬ������������Ϊֹ����������������������������Ĺ�ϵ��ͼ���� CO2���ܶ�Ϊ1.96g/L�����������λС����
��1��������CO2���������Ϊ
0.03
0.03
%����2������5.0g����ǰû�����ݲ�����ԭ����
NaOH+HCl�TNaCl+H2O
NaOH+HCl�TNaCl+H2O
���û�ѧ����ʽ��ʾ������3��������������CO2���������Ƕ��٣�
��4����������Ʒ��CO2����������Ƕ��٣�
��������1�������ж�����̼���������Ϊ0.03%�����Ծݴ˽����⣻
��2������ͼ���֪��ϡ�����Ⱥ���������������Һ��Ӧ���ٺ�̼���Ʒ�Ӧ�����ܿ��������������ʷ�Ӧ��ϡ�����������
��3�����ݲ��뷴Ӧ��ϡ�������Ȼ�������������ݷ�Ӧ�Ļ�ѧ����ʽ�Ϳ�������ɶ�����̼��������
��4�����ݶ�����̼���ܶȺ������������������̼�����������������������
��2������ͼ���֪��ϡ�����Ⱥ���������������Һ��Ӧ���ٺ�̼���Ʒ�Ӧ�����ܿ��������������ʷ�Ӧ��ϡ�����������
��3�����ݲ��뷴Ӧ��ϡ�������Ȼ�������������ݷ�Ӧ�Ļ�ѧ����ʽ�Ϳ�������ɶ�����̼��������
��4�����ݶ�����̼���ܶȺ������������������̼�����������������������
����⣺��1�������ж�����̼���������Ϊ0.03%��
��2���������Ⱥ���������������Һ������Ӧ�����Լ���5.0g����ǰû�в������壮�������ķ�Ӧ�Ļ�ѧ����ʽΪ��NaOH+HCl�TNaCl+H2O��
��3����ͼ�п���֪����̼���Ʒ�Ӧ�����������=��19.6g-5.0g����5%=0.73g
�����ɶ�����̼������Ϊx
Na2CO3+2HCl�T2NaCl+CO2��+H2O
73 44
0.73g x
=
��ã�x=0.44g
��4����������Ʒ��CO2����������ǣ�
��100%=4.48%
��3��������������CO2����������0.44g��
��4����������Ʒ��CO2�����������4.48%��
�ʴ�Ϊ����1��0.03%��
��2��NaOH+HCl�TNaCl+H2O��
��3��0.44g��
��4��4.48%��
��2���������Ⱥ���������������Һ������Ӧ�����Լ���5.0g����ǰû�в������壮�������ķ�Ӧ�Ļ�ѧ����ʽΪ��NaOH+HCl�TNaCl+H2O��
��3����ͼ�п���֪����̼���Ʒ�Ӧ�����������=��19.6g-5.0g����5%=0.73g
�����ɶ�����̼������Ϊx
Na2CO3+2HCl�T2NaCl+CO2��+H2O
73 44
0.73g x
| 73 |
| 0.73g |
| 44 |
| x |
��ã�x=0.44g
��4����������Ʒ��CO2����������ǣ�
| 0.44g��1.96g/L |
| 5L |
��3��������������CO2����������0.44g��
��4����������Ʒ��CO2�����������4.48%��
�ʴ�Ϊ����1��0.03%��
��2��NaOH+HCl�TNaCl+H2O��
��3��0.44g��
��4��4.48%��
�����������ѶȲ��Ǻܴ���Ҫ������ͼ������ݵķ��������ݻ�ѧ����ʽ���м��㣬����ѧ���ķ��������ͽ�������������

��ϰ��ϵ�д�
�����Ŀ
 СϣΪ�˲ⶨ���������������CO2������������������ʵ�飺���ռ�5L������������Ʒ������������Ʒ�м��������NaOH��Һ����ַ�Ӧ���۰ѷ�Ӧ�����Һת�Ƶ��ձ��У���������5%�����ᣬ������������Ϊֹ����������������������������Ĺ�ϵ��ͼ���� CO2���ܶ�Ϊ1.96g/L�����������λС������1������5.0g����ǰû�����ݲ�����ԭ����
СϣΪ�˲ⶨ���������������CO2������������������ʵ�飺���ռ�5L������������Ʒ������������Ʒ�м��������NaOH��Һ����ַ�Ӧ���۰ѷ�Ӧ�����Һת�Ƶ��ձ��У���������5%�����ᣬ������������Ϊֹ����������������������������Ĺ�ϵ��ͼ���� CO2���ܶ�Ϊ1.96g/L�����������λС������1������5.0g����ǰû�����ݲ�����ԭ���� СϣΪ�˲ⶨ���������������CO2������������������ʵ�飺���ռ�5L������������Ʒ������������Ʒ�м��������NaOH��Һ����ַ�Ӧ���۰ѷ�Ӧ�����Һת�Ƶ��ձ��У���������5%�����ᣬ������������Ϊֹ����������������������������Ĺ�ϵ��ͼ���� CO2���ܶ�Ϊ1.96g/L�����������λС����
СϣΪ�˲ⶨ���������������CO2������������������ʵ�飺���ռ�5L������������Ʒ������������Ʒ�м��������NaOH��Һ����ַ�Ӧ���۰ѷ�Ӧ�����Һת�Ƶ��ձ��У���������5%�����ᣬ������������Ϊֹ����������������������������Ĺ�ϵ��ͼ���� CO2���ܶ�Ϊ1.96g/L�����������λС����
