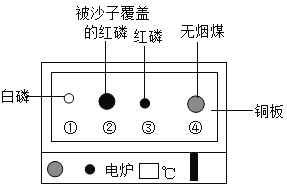
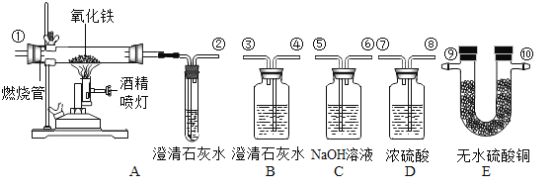
��Ŀ����
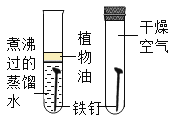
����Ŀ��ʵ����������ͼ��ʾװ�ÿ�����ȡijЩ���壬��ش��������⣺
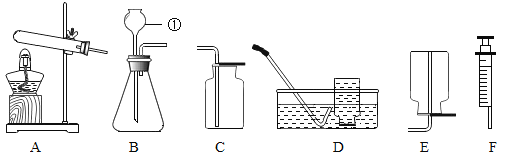
��1��д����Ţٵ��������ƣ�_____��
��2������Bװ�ÿ���ȡ�������塣װ��ҩƷǰ������ע����F���װ��B�������ԣ��������£�
������ƿ��ˮ��_____���γ�Һ�⣻
�ڽ�ע����F���ӵ�װ��B�ĵ��ܿڴ���
�ۻ�������ע�����Ļ������۲쵽_____����ʾװ��B�����������á�
��3��ʵ������װ��B��ȡ������̼���壬��Ӧ����ʽ��_____��
��4��ʵ���ҳ���30%�Ĺ���������Һ�Ͷ���������ȡ����������ˮ���ռ��������ⶨ����ƿ�������ĺ������ظ�ʵ��3�Ρ�ʵ���������£�
ʵ��1 | ʵ��2 | ʵ��3 | |
���������������%�� | 90.0 | 89.8 | 89.3 |
������ƽ�����������%�� | 89.7 | ||
ʵ���л����������������ܴﵽ100%����Ҫԭ�������_____��
��5��������NH3����һ�ּ�������ˮ�����壬ʵ���ҳ����ü����Ȼ�泥����壩�ͼ�ʯ�ң����壩�Ļ��������ȡ��������Ӧ�Ļ�ѧ����ʽΪ_____��ȡһƿ����Ӧѡ���װ����_____��������ţ�
���𰸡�����©�� ����©���¶�����Һ������ ���ܿ�������ð�� CaCO3+2HCl=CaCl2+H2O+CO2�� �����к���һ����ˮ���� 2NH4Cl+Ca��OH��2![]() CaCl2+2H2O+2NH3�� AE
CaCl2+2H2O+2NH3�� AE
��������
��1����Ţٵ����������dz���©����
��2��������ƿ��ˮ������©���¶�����Һ�������γ�Һ�⣻
�ۻ�������ע�����Ļ������۲쵽���ܿ�������ð����֤��װ�õ����������ã�
��3��̼��ƺ�ϡ���ᷴӦ�����Ȼ��ơ�ˮ�Ͷ�����̼����ѧ����ʽΪ��CaCO3+2HCl=CaCl2+H2O+CO2����
��4����Ϊ������ˮ���ռ��������лẬ��һ������ˮ����������ʵ���л����������������ܴﵽ100%����Ҫԭ������ǣ������к���һ����ˮ������
��5��ʵ���ҳ����ü����Ȼ�泥����壩�ͼ�ʯ�ң����壩�Ļ��������ȡ���������ڹ̡��̼����ͣ��ʺ���װ��A������װ�ã�������NH3����һ�ּ�������ˮ���ܶȱȿ���С�����壬�ʺ���װ��E���ռ�װ�ã��Ȼ�狀ͼ�ʯ���ڼ��ȵ������������Ȼ��ơ�ˮ�Ͱ�������ѧ����ʽΪ��2NH4Cl+Ca��OH��2![]() CaCl2+2H2O+2NH3����
CaCl2+2H2O+2NH3����

����Ŀ����һ�������£���һ���ܱ������ڷ���ij��Ӧ����÷�Ӧ�����и����ʵ����������ʾ������˵������ȷ���ǣ�������
���� | X | Y | Z | W |
��Ӧǰ����/g | 10 | 3 | 90 | 0 |
��Ӧ������/g | 3.2 | 3 | ���� | 3.2 |
A. W�����ǵ��� B. Y�����Ǵ���
C. �÷�Ӧ�ǷֽⷴӦ D. ��Ӧ��Z���ʵ�����Ϊ![]()
����Ŀ����������������Ҫ�Ļ���ԭ�ϡ�
��1����������_____������ӡ�����ԭ�ӡ������ӡ������ɵġ�
��2����������ˮ�Լ��ԣ�����Ϊ������ˮ��Ӧ�����˼��д�����ּ�Ļ�ѧʽ_____��
��3��NH4NO3 ��һ����Ҫ�ĵ��ʣ����� NH4NO3�е�Ԫ�صĻ��ϼ�_____����֪NH4NO3��KCl���������е�Ԫ�ص���������Ϊ28%����������KCl���������� Ϊ_____��
��4������ɼ����Ȼ�狀������������Һ��ʵ�鱨�档
ʵ�鲽�� | ʵ������ | ʵ����� |
______ | ______ | ______ |
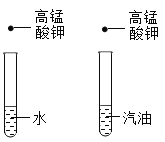
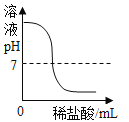
����Ŀ�������£����ձ��ڵļ����ʼ�������������������x���ʾ���������ʵ�������������ѡ����ͼ��������ǣ� ��
ѡ�� | ������ | ������ | Y������� |
A | ϡ����������� | �������� | ���ɳ��������� |
B | ˮ | ����� | ���ʵ��������� |
C | �������� | ����������Һ | ���������� |
D | �� | ϡ���� | ��Һ����Ԫ�ص����� |
A.AB.BC.CD.D
����Ŀ������Ϊ���������ڽ�200�������ʷ�б��㷺Ӧ�á������������ϻش�
���ʵ������Ϣ | ͭ | �� |
�ܶȣ�g/cm3�� | 8.92 | 2.70 |
�ؿ��к�������������/%�� | 0.007 | 7.73 |
�����ԣ������ĵ�����Ϊ100������ | 99 | 61 |
��1�����ĵ����Ա�ͭС��Ϊʲô��ѹ���߳�������_____��
��2�������Ƹ�ѹ����ʱ���治��Ҫ����Ե�壬�û�ѧ����ʽ��ʾԭ��_____��
��3���ɻ������Ӳ���ƶ��������Ƶ�ԭ��_____��