��Ŀ����
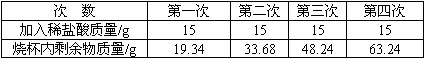
Ϊ�˲ⶨij��ʯ��ʯ��̼��Ƶ�����������ȡ5gʯ��ʯ��Ʒ�����ձ��У���60gϡ����ֳ��Ĵμ����ձ��У���ַ�Ӧ���ʵ���������±�(��Ʒ�е����ʲ������ᷴӦҲ������ˮ)��
��������������йؼ��㣺
(1)�ļ��η�Ӧ��������ʣ��_____________��
(2)ʯ��ʯ��Ʒ��̼��Ƶ�����������
(3)����ʵ����������ձ��ڵ������м���ʯ��ʯ�����ٲ������ݣ����ˡ�����Һ�����ʵ�����������(�����ȷ��0.1%)
(1)�ļ��η�Ӧ��������ʣ��_____________��
(2)ʯ��ʯ��Ʒ��̼��Ƶ�����������
(3)����ʵ����������ձ��ڵ������м���ʯ��ʯ�����ٲ������ݣ����ˡ�����Һ�����ʵ�����������(�����ȷ��0.1%)
(1)������
(2)�⣺��5gʯ��ʯ��CaCO3������Ϊx��
CaCO3+2HCl==CaCl2+H2O+CO2��
��100��������������������44
��x��������������������1.76g
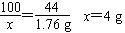
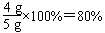
(3)��60g������ȫ��Ӧ��CaCO3����Ϊy�����ɵ�CaCl2����Ϊz��������CO2����Ϊm��
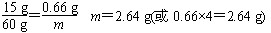
(��������һ�βμӷ�Ӧ��CaCO3������1.5g��1.5g��4��6g��ͨ����һ�η�Ӧ�����������������������Ϊ7.3%)
CaCO3+2HCl==CaCl2+H2O+CO2��
��100��������111��������44
��y������������z��������2.64g
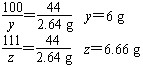
��Һ������6g��60g��2.64g��63.36g
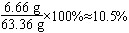
(2)�⣺��5gʯ��ʯ��CaCO3������Ϊx��
CaCO3+2HCl==CaCl2+H2O+CO2��
��100��������������������44
��x��������������������1.76g
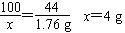
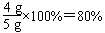
(3)��60g������ȫ��Ӧ��CaCO3����Ϊy�����ɵ�CaCl2����Ϊz��������CO2����Ϊm��
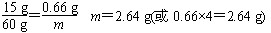
(��������һ�βμӷ�Ӧ��CaCO3������1.5g��1.5g��4��6g��ͨ����һ�η�Ӧ�����������������������Ϊ7.3%)
CaCO3+2HCl==CaCl2+H2O+CO2��
��100��������111��������44
��y������������z��������2.64g
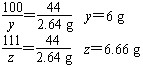
��Һ������6g��60g��2.64g��63.36g
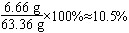

��ϰ��ϵ�д�
�����Ŀ
Ϊ�˲ⶨij��ʯ��ʯ��̼��Ƶ�����������ȡ5gʯ��ʯ��Ʒ�����ձ��У���60gϡ����ֳ��Ĵμ����ձ��У���ַ�Ӧ���ʵ���������±�����Ʒ�е����ʲ������ᷴӦҲ������ˮ��
��������������йؼ��㣺
��1���ļ��η�Ӧ��������ʣ�� ��
��2��ʯ��ʯ��Ʒ��̼��Ƶ�����������
��3������ʵ����������ձ��ڵ������м���ʯ��ʯ�����ٲ������ݣ����ˣ�����Һ�����ʵ������������������ȷ��0.1%��
| �Ρ��� | ��һ�� | �ڶ��� | ������ | ���Ĵ� |
| ����ϡ��������/g | 15 | 15 | 15 | 15 |
| �ձ���ʣ��������/g | 19.34 | 33.68 | 48.24 | 63.24 |
��1���ļ��η�Ӧ��������ʣ��
��2��ʯ��ʯ��Ʒ��̼��Ƶ�����������
��3������ʵ����������ձ��ڵ������м���ʯ��ʯ�����ٲ������ݣ����ˣ�����Һ�����ʵ������������������ȷ��0.1%��
