��Ŀ����
ÿ���3��22��Ϊ����ˮ�գ�ˮ��Դ�Ŀɳ��������ѳ�Ϊȫ�������ǵĹ�ͬ��ע�Ļ��⣮�ƺ����л������ҡ�������ҹ��������ε��ص����֮һ�����ƺ�ˮϵͼ���ش��������⣮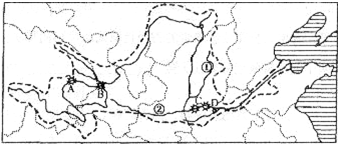
��1���ƺӷ�Դ��
��2��д��ͼ��֧�����Ƣ���
��3��ABCD�ĸ�ˮ��վ�У�A��
��4���ƺ���Ѵ��Ҫ������
��5���ƺӵľ�����С�ڳ�������Ȼԭ����Ҫ��
��6������Ϊ����ƺ�����ȱˮ����Ҫ��ʩ����Щ��
�������ƺ�Դ���տ���ɽ�������ຣ���Ĵ���9ʡ����ע�벳�������ɹ��������ĺӿ�����Ϊ���Σ��ӿڵ����ϵľ��Ͻ�Ϊ���Σ����Ͻ�����Ϊ���Σ��ƺ������ڵ�һ�������ݽ��紦����������ˮ�ܷḻ���ƺ���������������ԭ������ˮ����ʧ���أ�������ɳ�����������ڻ���ƽԭ�ϣ��Ӵ���̹��ˮ����������ɳ�����ٻ���ʹ�Ӵ�̧�ߣ���Ϊ���������ĵ��Ϻӣ�
����⣺��1���ƺӷ�Դ����ظ�ԭ�İ��տ���ɽ��
��2����ͼ�п������ƺӵ���Ҫ֧�����Ƿںӣ�����μ�ӣ�
��3����ͼ�п�����λ�ڻƺ����ε�ˮ��վA������Ͽ��λ�����ε�D��С�˵ף�ˮ��վ�ֲ��Ĺ�ͬ�ص��Ƕ�λ�ڽ��ݽ��紦����ô���������ˮ�ܷḻ��
��4�����ڽϸ�γ�ȺӶ��ﶬ���ڷⶳ�磬�������ڽⶳ�٣������γɱ��ӣ�������γ�ȵĺӵ���ˮ�����º�ˮ���ģ����Իƺ���Ѵ��Ҫ�����ڴӵ�γ�������γ�ȵĺӶΣ�
��5���ƺ�����Ľ�ˮ��С�ڳ����������Իƺӵľ�����С�ڳ�����
��6���ƺ�����ȱˮ����Ҫ��ʩ����ˮ��������ˮ�⣬��Լ��ˮ������ˮ��Ⱦ��������ˮ�ȣ�
�ʴ�Ϊ����1���ຣ�����տ���ɽ����2���ںӣ�μ�ӣ���3������Ͽ��С�˵ף����ݽ��紦����4���ӵ�γ�������γ�ȺӶΣ���5���ƺ�����Ľ�ˮ��С�ڳ�������6����ˮ��������ˮ�⣬��Լ��ˮ������ˮ��Ⱦ��������ˮ�ȣ�
��2����ͼ�п������ƺӵ���Ҫ֧�����Ƿںӣ�����μ�ӣ�
��3����ͼ�п�����λ�ڻƺ����ε�ˮ��վA������Ͽ��λ�����ε�D��С�˵ף�ˮ��վ�ֲ��Ĺ�ͬ�ص��Ƕ�λ�ڽ��ݽ��紦����ô���������ˮ�ܷḻ��
��4�����ڽϸ�γ�ȺӶ��ﶬ���ڷⶳ�磬�������ڽⶳ�٣������γɱ��ӣ�������γ�ȵĺӵ���ˮ�����º�ˮ���ģ����Իƺ���Ѵ��Ҫ�����ڴӵ�γ�������γ�ȵĺӶΣ�
��5���ƺ�����Ľ�ˮ��С�ڳ����������Իƺӵľ�����С�ڳ�����
��6���ƺ�����ȱˮ����Ҫ��ʩ����ˮ��������ˮ�⣬��Լ��ˮ������ˮ��Ⱦ��������ˮ�ȣ�
�ʴ�Ϊ����1���ຣ�����տ���ɽ����2���ںӣ�μ�ӣ���3������Ͽ��С�˵ף����ݽ��紦����4���ӵ�γ�������γ�ȺӶΣ���5���ƺ�����Ľ�ˮ��С�ڳ�������6����ˮ��������ˮ�⣬��Լ��ˮ������ˮ��Ⱦ��������ˮ�ȣ�
������������Ҫ����ƺӵĸſ���˵��ͼ��Bˮ��վ�����ƣ�

��ϰ��ϵ�д�
 �����������һ��һ��ϵ�д�
�����������һ��һ��ϵ�д� Ӧ������ҵ��ϵ�д�
Ӧ������ҵ��ϵ�д�
�����Ŀ
ÿ���3��22��Ϊ����ˮ�գ�ּ�ڻ����ڵĽ�ˮ��ʶ����ǿˮ��Դ������2012��3��22�������Ϲ�ȷ���ĵڶ�ʮ��������ˮ�ա�����������ˮ�յ������ǡ�ˮ ����ʳ��ȫ�����ݴ˻ش����⣺
�ҹ�ˮ��Դʱ�շֲ����������Ӹ�����Ťת�ҹ�ˮ��Դ�����ֲ����ز�������ĺ�ΰˮ�������ǣ�������
�ҹ�ˮ��Դʱ�շֲ����������Ӹ�����Ťת�ҹ�ˮ��Դ�����ֲ����ز�������ĺ�ΰˮ�������ǣ�������
| A����Ͽ���� | B��С�˵�ˮ������ | C����ˮ���� | D�����Ƽ��� |
ÿ���3��22��Ϊ����ˮ�գ�3��22-28��Ϊ�й�ˮ�ܣ�ּ�ڽ�һ�����ȫ������ˮ����ϧˮ������ˮ��ˮ�ǻ���ʶ���ٽ�ˮ��Դ�Ŀ��������á�����������2013������ˮ�յ��������⡰ˮ��������WaterCooperation�����ҹ�����2013�ꡰ����ˮ�ա��͡��й�ˮ�ܡ������������Ϊ��������
| A����Լ����ˮ��Դ������������̬���� | B���ϸ����ˮ��Դ���ƽ�ˮ���¿�Խ | C���ϸ�ˮ��Դ���������Ͽɳ�����չ | D����ʵ��ѧ��չ�ۣ���Լ����ˮ��Դ |
 ��2006?���ݣ��Ķ����²��ϣ��ش��������⣮
��2006?���ݣ��Ķ����²��ϣ��ش��������⣮