��Ŀ����
ij�ӵ������м��������������ŷŵĹ�ҵ��ˮ�У�����K+��Ag+��Fe3+��C1?��OH?��NO3-�������ӣ��׳��ķ�ˮ���Գʼ��ԣ��ʼ׳���ˮ���������������� �� �� ���ҳ��ķ�ˮ�к��������������ӣ����ڸ������ķ�ˮ���ɲ�ȡ�������ַ�����������
��1������ڷ�ˮ�м�һ���� ��ѡ�����̿�����ۣ������Է��� ��Ӧ�����ӷ���ʽ�� �������ܹ��������еĽ��� ����д����Ԫ�ط��ţ���
��2�����׳����ҳ��ķ�ˮ���ʵ��ı�����ϣ�����ʹ��ˮ�е�ijЩ����ת��Ϊ������д���������ӷ���ʽ��
�� �������˺�ķ�ˮ��Ҫ�� ������������ũ�
��1������ڷ�ˮ�м�һ����
��2�����׳����ҳ��ķ�ˮ���ʵ��ı�����ϣ�����ʹ��ˮ�е�ijЩ����ת��Ϊ������д���������ӷ���ʽ��
���㣺���������ӵļ���,���������ӵļ���
ר�⣺���ʼ��������
�������׳���ˮ�ʼ��ԣ�����Һ�к��д�����OH-����OH-���ӷ�Ӧ��Ag+��Fe3+���ܴ������棬������Һ�����Կ�֪�׳���ˮ��Ӧ����K+���ҳ��к���Ag+��Fe3+������Ag+��Fe3+��Ӧ��Cl-��OH-���ܹ��棬������Һ�����Կ�֪�ҳ�����NO3-��
�ɴ˿�֪���׳�����K+��Cl-��OH-���ҳ�����Ag+��Fe3+��NO3-�����������к��е�������ɼ����ʽ����⣮
�ɴ˿�֪���׳�����K+��Cl-��OH-���ҳ�����Ag+��Fe3+��NO3-�����������к��е�������ɼ����ʽ����⣮
���
�⣺��1���׳���ˮ�ʼ��ԣ�����Һ�к��д�����OH-����OH-���ӷ�Ӧ��Ag+��Fe3+���ܴ������棬
������Һ�����Կ�֪�׳���ˮ��Ӧ����K+���ҳ��к���Ag+��Fe3+������Ag+��Fe3+��Ӧ��Cl-��OH-���ܹ��棬������Һ�����Կ�֪�ҳ�����NO3-��
�ɴ˿�֪���׳�����K+��Cl-��OH-���ҳ�����Ag+��Fe3+��NO3-���ʴ�Ϊ��K+��Cl-��OH-��
��2�����еĽ���������K+��Ag+��Fe3+�����뵥��Fe�������û���Ag����Ӧ�����ӷ���ʽΪ��Fe+2Ag+=2Ag+Fe2+��
�ʴ�Ϊ�����ۣ��û���Fe+2Ag+=2Ag+Fe2+��Ag��
��3�����׳����ҳ��ķ�ˮ���ʵ��ı�����ϣ�������AgCl��Fe��OH��3��������Ӧ���ӷ���ʽΪ��Ag++Cl-=AgCl����Fe3++3OH-=Fe��OH��3����
���ɳ�����������Ag+��Fe3+��Cl-��OH-�ȣ������˺�ķ�ˮ��ҪKNO3����������ʹ�ã�
�ʴ�Ϊ��Ag++Cl-=AgCl����Fe3++3OH-=Fe��OH��3����KNO3��
������Һ�����Կ�֪�׳���ˮ��Ӧ����K+���ҳ��к���Ag+��Fe3+������Ag+��Fe3+��Ӧ��Cl-��OH-���ܹ��棬������Һ�����Կ�֪�ҳ�����NO3-��
�ɴ˿�֪���׳�����K+��Cl-��OH-���ҳ�����Ag+��Fe3+��NO3-���ʴ�Ϊ��K+��Cl-��OH-��
��2�����еĽ���������K+��Ag+��Fe3+�����뵥��Fe�������û���Ag����Ӧ�����ӷ���ʽΪ��Fe+2Ag+=2Ag+Fe2+��
�ʴ�Ϊ�����ۣ��û���Fe+2Ag+=2Ag+Fe2+��Ag��
��3�����׳����ҳ��ķ�ˮ���ʵ��ı�����ϣ�������AgCl��Fe��OH��3��������Ӧ���ӷ���ʽΪ��Ag++Cl-=AgCl����Fe3++3OH-=Fe��OH��3����
���ɳ�����������Ag+��Fe3+��Cl-��OH-�ȣ������˺�ķ�ˮ��ҪKNO3����������ʹ�ã�
�ʴ�Ϊ��Ag++Cl-=AgCl����Fe3++3OH-=Fe��OH��3����KNO3��
���������⿼�����ӹ������⣬��Ŀ�ѶȲ���ע���жϼ��ҳ���ˮ�ɷֵĽǶ��Լ����ӵ����ʣ�

��ϰ��ϵ�д�
�����Ŀ
������ͼʵ��װ���������Ӧʵ����ǣ�������


| A��װ�âٲⶨ��ѧ��Ӧ���� |
| B��װ�â���ȡ���������������� |
| C��װ�â�����Ʒ�����п |
| D��װ�â�ϡ��Ũ���� |



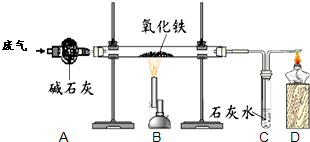 ij����С���ͬѧ�ռ��˺�ˮ������һ����̼�Ͷ�����̼�ķ�����Ϊȷ�����ַ����д���CO��������ʵ���Ұ���ͼ��ʾװ�ý���ʵ�顲����ͨ��װ��A�ٶȺ����������ڴ˴������ķ�Ӧ��ȫ����ʯ�ң�CaO��NaOH�Ļ�����������
ij����С���ͬѧ�ռ��˺�ˮ������һ����̼�Ͷ�����̼�ķ�����Ϊȷ�����ַ����д���CO��������ʵ���Ұ���ͼ��ʾװ�ý���ʵ�顲����ͨ��װ��A�ٶȺ����������ڴ˴������ķ�Ӧ��ȫ����ʯ�ң�CaO��NaOH�Ļ����������� ��ͼ��ʾװ�ý���ʵ�飨ͼ������̨������������ȥ�����ڢ��м����Լ���������Ƥ����������ֹˮ�У�����������ð����һ��ʱ���ر�ֹˮ�У�����Һ����������Һ����ɫ��Ϊ���ǣ���������ʵ������Ģ�͢���Ӧ������Լ��ǣ�������
��ͼ��ʾװ�ý���ʵ�飨ͼ������̨������������ȥ�����ڢ��м����Լ���������Ƥ����������ֹˮ�У�����������ð����һ��ʱ���ر�ֹˮ�У�����Һ����������Һ����ɫ��Ϊ���ǣ���������ʵ������Ģ�͢���Ӧ������Լ��ǣ�������