��Ŀ����
13�� �Ȼ���S2Cl2����һ�ֻƺ�ɫҺ�壬�д̼��ԡ���Ϣ�Զ�����۵�Ϊ-80�棬�е�137.1�森�ڿ�����ǿ�ҷ��̣�����ˮ����ˮ�ⷴӦ������ʹ������������ʹ�����ı������ȷ�ճ���Ӳ�IJ������ܣ������ڵ�����ͨ��������������S2Cl2����ͼ��ʵ������S��Cl2�Ʊ�S2Cl2��װ�ã��г�װ�á�����װ�þ�����ȥ����
�Ȼ���S2Cl2����һ�ֻƺ�ɫҺ�壬�д̼��ԡ���Ϣ�Զ�����۵�Ϊ-80�棬�е�137.1�森�ڿ�����ǿ�ҷ��̣�����ˮ����ˮ�ⷴӦ������ʹ������������ʹ�����ı������ȷ�ճ���Ӳ�IJ������ܣ������ڵ�����ͨ��������������S2Cl2����ͼ��ʵ������S��Cl2�Ʊ�S2Cl2��װ�ã��г�װ�á�����װ�þ�����ȥ������1����֪S2Cl2���ӽṹ��H2O2���ƣ���д��S2Cl2�ĵ���ʽ
 ��
����2��װ��a��Ӧ���Լ�ΪŨ���ᣬ������Ϊ����Cl2��װ��cΪ��ˮƽ���÷�ֹ���ڵ���������ܣ�
��3����ʵ��IJ���˳��ӦΪ�ڢۢ٢ݢܣ���ۢڢ٢ݢܣ� ������ű�ʾ����
�ټ���װ��c ��ͨ��Cl2 ��ͨ����ˮ ��ֹͣͨCl2 ��ֹͣ����װ��c
��4��fװ����Ӧ���õ��Լ�Ϊ��ʯ�ң�������Ϊ����Cl2β������ֹ��Ⱦ��������ֹ�����е�ˮ����ʹS2Cl2ˮ�⣮
��5����֪S2Cl2ˮ��ʱ��ֻ��һ��Ԫ�صĻ��ϼ۷����˱仯���ұ������ͱ���ԭ�ĸ�Ԫ�ص����ʵ���֮��Ϊ1��3����д���÷�Ӧ�Ļ�ѧ����ʽ2S2Cl2+2H2O=SO2��+3S��+4HCl��
���� ��1��S2Cl2���ӽṹ��H2O2���ƣ�S2Cl2��������ԭ������ԭ��֮���γ�1�Թ��õ��Ӷԣ���ԭ������ԭ��֮���γ�1�Թ��õ��Ӷԣ�
��2��������������״ȷ�����ƣ�����Ϣ��֪S2Cl2��ˮˮ�⣬����b�е�����Ӧ�ø����Ũ������
��3������֮ǰ��ͨ����ˮ������ʼ���ɵ�S2Cl2������ȴҺ���������ֹͣ���Ⱥ�ֹͣͨ������ƽ��������ѹǿ����ֹ����Σ�գ�
��4��fװ��ʢ�ż�ʯ�ң�����Cl2β������ֹ�����е�ˮ������e�У�
��5��ֻ����Ԫ�ػ��ϼ۷����仯�����ݱ������ͱ���ԭ�ĸ�Ԫ�ص����ʵ���֮��Ϊ1��3��д��
��� �⣺��1��S2Cl2���ӽṹ��H2O2���ƣ�S2Cl2��������ԭ������ԭ��֮���γ�1�Թ��õ��Ӷԣ���ԭ������ԭ��֮���γ�1�Թ��õ��Ӷԣ�S2Cl2����ʽΪ ���ʴ�Ϊ��
���ʴ�Ϊ��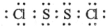 ��
��
��2������Ϣ��֪S2Cl2��ˮˮ�⣬����b�е�����Ӧ�ø��Ũ���������ˮ��������������Ӧ������a��Ӧ���Լ�ΪŨ���ᣬ������Ϊ����������װ��cΪ��ˮƽ������Ϊ��ֹ���ڵ���������ܣ�
�ʴ�Ϊ��ŨH2SO4������Cl2����ֹ���ڵ���������ܣ�
��3������֮ǰ��ͨ����ˮ������ʼ���ɵ�S2Cl2������ȴҺ���������ֹͣ���Ⱥ�ֹͣͨ������ƽ��������ѹǿ����ֹ����Σ�գ�����ʵ�����˳��Ϊ�ڢۢ٢ݢܣ���ۢڢ٢ݢܣ���
�ʴ�Ϊ���ڢۢ٢ݢܣ���ۢڢ٢ݢܣ���
��4�������ж���Ϊ�������壬fװ��ʢ�ż�ʯ�ң�����Cl2β������ֹ��Ⱦ��������ֹ�����е�ˮ������e��ʹS2Cl2ˮ�⣬
�ʴ�Ϊ����ʯ�ң�����Cl2β������ֹ��Ⱦ��������ֹ�����е�ˮ����ʹS2Cl2ˮ�⣻
��5��ֻ����Ԫ�ػ��ϼ۷����仯����Ӧ����Ԫ�ش�+1�۲��ֱ�Ϊ+4�ۣ�����������ΪSO2�����ֱ�Ϊ0�ۣ��û�ԭ����ΪS���ұ������ͱ���ԭ�����ʵ���֮��Ϊ1��3����ѧ����ʽΪ2S2Cl2+2H2O=SO2��+3S��+4HCl��
�ʴ�Ϊ��2S2Cl2+2H2O=SO2��+3S��+4HCl��
���� ���⿼���Ʊ�ʵ�鷽������ƣ�Ϊ��Ƶ���㣬�����Ʊ�ʵ��ԭ����ʵ��װ�õ����á�ϰ���е���ϢΪ���Ĺؼ������ط�����ʵ���������ۺϿ��飬��Ŀ�Ѷ��еȣ�


��֪���ٽ���Һ���е���������Ҫ��H+��Co2+��Fe2+��Mn2+��Ca2+��Mg2+��Al3+�ȣ�
�ڲ���������������������ʽ����ʱ��Һ��pH���±���
| ������ | Fe��OH��3 | Fe��OH��2 | Co��OH��2 | Al��OH��3 | Mn��OH��2 |
| ��ȫ������pH | 3.7 | 9.6 | 9.2 | 5.2 | 9.8 |
��2����ȡNaClO3���Խ�����ͨ�뵽�ȵ�Ũ����������Һ���÷�Ӧ�����ӷ���ʽΪ3Cl2+6OH-$\frac{\underline{\;\;��\;\;}}{\;}$ClO3-+5Cl-+3H2O��ʵ����Ҫ��ȡ10.65��NaClO3����Ҫ�������ɵ��ʳ��ˮ���ɣ��������Ƿ�Ӧ�����е���ʧ����ͬʱ���ɵ����������Ϊ6.72����״������
��3����ȡ���Խ������ӵ���ȡ����pH�Ĺ�ϵ��ͼ2��ʾ����Һ���м�����ȡ���������dz�ȥMn2+��ʹ����ȡ�����˵�pH��B����ѡ����ţ���
A���ӽ�2.0 B���ӽ�3.0 C���ӽ�5.0
��4�������ơ�þ���ǽ���Һ��Ca2+��Mg2+ת��ΪMgF2��CaF2��������֪Ksp��MgF2��=7.35��10-11��Ksp��CaF2��=1.05��10-10�����������NaF��������Һc��Mg2+��/c��Ca2+��=0.7��
��5����ҵ���ð�ˮ���շ����е�SO2����֪NH3•H2O�ĵ���ƽ�ⳣ��K=1.8��10-5mol•L��H2SO3�ĵ���ƽ�ⳣ��K1=1.2��10-2mol•L-4��K2=1.3��10-8mol•L-1����ͨ������Ĺ����У�
�ٵ�ǡ���γ�����ʱ����Һ������Ũ�ȵĴ�С��ϵΪc��NH4+����c��SO32-����c��OH-����c��HSO3-����c��H+����
�ڵ�ǡ���γ���ʽ��ʱ����������NaOH��Һ����Ӧ�����ӷ���ʽΪHSO3-+OH-=SO32-+H2O��
| A�� | ���Թ��е�ͭ��Ũ������ȣ������Թܵײ��а�ɫ���岢������������ɫ���ʣ��˰�ɫ����Ϊ����ͭ����ɫ����Ϊ����ͭ | |
| B�� | SO2ͨ��������Fe��NO3��3ϡ��Һ�У���Һ���ػ�ɫ��Ϊdz��ɫ���������ֱ���ػ�ɫ��˵�������ԣ�HNO3��ϡ���Fe3+ | |
| C�� | ����һ�����ʵ���Ũ����Һʱ����Ũ��ƫ�ͣ���������������Һ���õ�����ƿ����δ���� | |
| D�� | Ũ�����ڹ��������±�ƣ�˵��Ũ����ȶ������ɵ���ɫ����������Ũ���� |
| A�� | ������к���0.05molFeԪ�� | |
| B�� | ��������Һ�����ʵ�����������63% | |
| C�� | ������NaOH��Һ�����������450mL | |
| D�� | ��Ӧ��HNO3��������������������NO |
 ������Ϊ�÷����Ƿ�Ϊƽ���η��ӣ�����ǡ�����
������Ϊ�÷����Ƿ�Ϊƽ���η��ӣ�����ǡ�����

 ��CH3CH2CH2CHO��
��CH3CH2CH2CHO�� ��
��
 ��Ȼ����ڷḻ��̼�������衢�ס�����Ԫ�أ����ǿ��γɵ��ʼ���������Ҫ��ش��������⣺
��Ȼ����ڷḻ��̼�������衢�ס�����Ԫ�أ����ǿ��γɵ��ʼ���������Ҫ��ش��������⣺ ��֪���������ʹ���������Һ��ɫ������ͼװ�ã�����װ��û����������֤ij���������ͬʱ����SO2��CO2��
��֪���������ʹ���������Һ��ɫ������ͼװ�ã�����װ��û����������֤ij���������ͬʱ����SO2��CO2��