��Ŀ����
ʵ���л������ﷴӦ��ͼ���ݴ˻ش��������⣺

��1����ϩ�ĽṹʽΪ�� ��
��2��д����Ӧ�ڢۢܵĻ�ѧ����ʽ��
�� ����Ӧ���ͣ� ��
�� ����Ӧ���ͣ� ��
�� ����Ӧ���ͣ� ��
��3��ʵ���ҳ�������װ����ȡ����������ͼ2������ش�������⣺
��Ũ����������� ��
�ڱ���̼������Һ����Ҫ������ ��
����Ҫ���Ƶõ������������������Ӧ���õIJ����� ��
�ܽ��и�ʵ��ʱ��������Թܼ��м��뼸�����Ƭ����Ŀ���� ��
���ڴ��Թ�������һ���������Ҵ��������Ũ����Ļ��Һ�� �����ǣ������Թ��м���һ������ ��Ȼ����������� ����ȴ���ټ���һ������ ��������ʹ֮��Ͼ��ȣ�

��1����ϩ�ĽṹʽΪ��
��2��д����Ӧ�ڢۢܵĻ�ѧ����ʽ��
��
��
��
��3��ʵ���ҳ�������װ����ȡ����������ͼ2������ش�������⣺
��Ũ�����������
�ڱ���̼������Һ����Ҫ������
����Ҫ���Ƶõ������������������Ӧ���õIJ�����
�ܽ��и�ʵ��ʱ��������Թܼ��м��뼸�����Ƭ����Ŀ����
���ڴ��Թ�������һ���������Ҵ��������Ũ����Ļ��Һ�� �����ǣ������Թ��м���һ������
���㣺�л���ĺϳ�,������������ȡ
ר�⣺ʵ����,�л���Ļ�ѧ���ʼ��ƶ�
������CH2=CH2��ˮ�����ӳɷ�Ӧ����CH3CH2OH��CH3CH2OH��ͭ�����������������±�������������CH3CHO����C��CH3CHO��CH3CH2OH��D����������Ӧ����������������D��CH3COOH����ϩ�����Ӿ۷�Ӧ���� ��ʵ������ȡ��������������Ũ�����������ú���ˮ���ã������Ƭ�����У��ñ���̼������Һ�����ᷴӦ��ȥ���ᡢͬʱ���������������ܽ�ȣ����ڷֲ㣬�÷�Һ�����������������������Ϊ��ֹ��Һ�ɽ���Ӧ�ȼ����Ҵ���Ȼ���ڼ���Ũ��������ᣬ�ݴ˴��⣮
��ʵ������ȡ��������������Ũ�����������ú���ˮ���ã������Ƭ�����У��ñ���̼������Һ�����ᷴӦ��ȥ���ᡢͬʱ���������������ܽ�ȣ����ڷֲ㣬�÷�Һ�����������������������Ϊ��ֹ��Һ�ɽ���Ӧ�ȼ����Ҵ���Ȼ���ڼ���Ũ��������ᣬ�ݴ˴��⣮
 ��ʵ������ȡ��������������Ũ�����������ú���ˮ���ã������Ƭ�����У��ñ���̼������Һ�����ᷴӦ��ȥ���ᡢͬʱ���������������ܽ�ȣ����ڷֲ㣬�÷�Һ�����������������������Ϊ��ֹ��Һ�ɽ���Ӧ�ȼ����Ҵ���Ȼ���ڼ���Ũ��������ᣬ�ݴ˴��⣮
��ʵ������ȡ��������������Ũ�����������ú���ˮ���ã������Ƭ�����У��ñ���̼������Һ�����ᷴӦ��ȥ���ᡢͬʱ���������������ܽ�ȣ����ڷֲ㣬�÷�Һ�����������������������Ϊ��ֹ��Һ�ɽ���Ӧ�ȼ����Ҵ���Ȼ���ڼ���Ũ��������ᣬ�ݴ˴��⣮���
�⣺CH2=CH2��ˮ�����ӳɷ�Ӧ����CH3CH2OH��CH3CH2OH��ͭ�����������������±�������������CH3CHO����C��CH3CHO��CH3CH2OH��D����������Ӧ����������������D��CH3COOH����ϩ�����Ӿ۷�Ӧ���� ��
��
��1����ϩ�ĽṹʽΪ��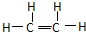 ��
��
�ʴ�Ϊ��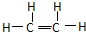 ��
��
��2�����Ҵ��ɱ�������Ϊ��ȩ����2CH3CH2OH+O2
2CH3CHO+2H2O���÷�Ӧ����������Ӧ��
�ʴ�Ϊ��2CH3CH2OH+O2
2CH3CHO+2H2O��������Ӧ��
����ϩ�����Ӿ۷�Ӧ�õ�����ϩ���� ���÷�Ӧ���ڼӾ۷�Ӧ��
���÷�Ӧ���ڼӾ۷�Ӧ��
�ʴ�Ϊ�� ���Ӿ۷�Ӧ��
���Ӿ۷�Ӧ��
���Ҵ������ᷢ��������Ӧ����������������CH3COOH+CH3CH2OH
CH3COOCH2CH3+H2O���÷�ӦΪȡ����Ӧ��
�ʴ�Ϊ��CH3COOH+CH3CH2OH
CH3COOCH2CH3+H2O��ȡ����Ӧ��
��3�����������Ҵ�����������Ӧ����Ũ�������������÷�ӦΪ���淴Ӧ��Ũ������ˮ����ƽ���������������������ƶ���Ũ���������Ϊ��������ˮ����
�ʴ�Ϊ����������ˮ����
���Ʊ���������ʱ���ñ���̼������Һ��Ŀ�����кͻӷ����������ᣬʹ֮ת��Ϊ����������ˮ�У�������������������ζ���ܽ�ӷ��������Ҵ�����������������ˮ�е��ܽ�ȣ����ڷֲ�õ�����
�ʴ�Ϊ���к����ᡢ�ܽ��Ҵ������������������ܽ�ȣ������ڷֲ㣻
����������������ˮ��Һ�ֲ㣬����Ҫ���Ƶõ������������������Ӧ���õIJ����Ƿ�Һ��
�ʴ�Ϊ����Һ��
�ܽ��и�ʵ��ʱ��������Թܼ��м��뼸�����Ƭ����Ŀ���Ƿ�ֹ��Һ���У�
�ʴ�Ϊ����ֹ��Һ���У�
��Ũ�����ܶȱ�ˮ������ˮ�ų��������ȣ�Ϊ��ֹ��Һ�ɽ�������ҩƷʱӦ�����Թ��м���һ�������Ҵ���Ȼ��ӱ����Թܽ�Ũ�������������Թܣ���ȴ���ټ���һ���������ᣬ
�ʴ�Ϊ���Ҵ���Ũ������
 ��
����1����ϩ�ĽṹʽΪ��
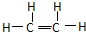 ��
���ʴ�Ϊ��
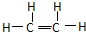 ��
����2�����Ҵ��ɱ�������Ϊ��ȩ����2CH3CH2OH+O2
| Cu |
| �� |
�ʴ�Ϊ��2CH3CH2OH+O2
| Cu |
| �� |
����ϩ�����Ӿ۷�Ӧ�õ�����ϩ����
 ���÷�Ӧ���ڼӾ۷�Ӧ��
���÷�Ӧ���ڼӾ۷�Ӧ���ʴ�Ϊ��
 ���Ӿ۷�Ӧ��
���Ӿ۷�Ӧ�����Ҵ������ᷢ��������Ӧ����������������CH3COOH+CH3CH2OH
| ŨH2SO4 |
| �� |
�ʴ�Ϊ��CH3COOH+CH3CH2OH
| ŨH2SO4 |
| �� |
��3�����������Ҵ�����������Ӧ����Ũ�������������÷�ӦΪ���淴Ӧ��Ũ������ˮ����ƽ���������������������ƶ���Ũ���������Ϊ��������ˮ����
�ʴ�Ϊ����������ˮ����
���Ʊ���������ʱ���ñ���̼������Һ��Ŀ�����кͻӷ����������ᣬʹ֮ת��Ϊ����������ˮ�У�������������������ζ���ܽ�ӷ��������Ҵ�����������������ˮ�е��ܽ�ȣ����ڷֲ�õ�����
�ʴ�Ϊ���к����ᡢ�ܽ��Ҵ������������������ܽ�ȣ������ڷֲ㣻
����������������ˮ��Һ�ֲ㣬����Ҫ���Ƶõ������������������Ӧ���õIJ����Ƿ�Һ��
�ʴ�Ϊ����Һ��
�ܽ��и�ʵ��ʱ��������Թܼ��м��뼸�����Ƭ����Ŀ���Ƿ�ֹ��Һ���У�
�ʴ�Ϊ����ֹ��Һ���У�
��Ũ�����ܶȱ�ˮ������ˮ�ų��������ȣ�Ϊ��ֹ��Һ�ɽ�������ҩƷʱӦ�����Թ��м���һ�������Ҵ���Ȼ��ӱ����Թܽ�Ũ�������������Թܣ���ȴ���ټ���һ���������ᣬ
�ʴ�Ϊ���Ҵ���Ũ������
������������Ҫ�������л���Ļ�ѧ���ʺͻ�ѧ����ʽ����д����Ŀ�ѶȲ����漰�������������Ʊ���Ũ���ᡢ����̼������Һ�������Լ�������Ӧ�Ļ���������ʱע���л�����֪ʶ��������ã�

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
��Cl2��������ˮ�в���CHCl3���к�±���������������Ƿ��֣���ClO2��������ˮ�������±����������˵����ȷ�ģ�������
| A��������Ч�ʣ��Ե�λ����õ��ӵ���Ŀ����������5�� |
| B��ClO2��Cl2ϡ��Һ���ڻ������������й���Ч���ص㣬���������κ�Σ�� |
| C��ClO2�ڳ�����Ϊ���壬����Ȼ����������ת��ΪCl2 |
| D���Ʊ�ClO2�ķ�Ӧ�ɱ�ʾΪ��Na2SO3+2NaClO3+H2SO4�T2Na2SO4+2ClO2��+H2O |
���������Һ�����������¿���������������Ӧ�����ӷ���ʽ���£�δ��ƽ����
MnO4-+ Fe2++ H+= Mn2++ Fe3++ H2O����˵����ȷ���ǣ�������
MnO4-+ Fe2++ H+= Mn2++ Fe3++ H2O����˵����ȷ���ǣ�������
| A��MnO4-����������Fe3+�ǻ�ԭ���� |
| B��Mn2+�Ļ�ԭ��ǿ��Fe2+ |
| C�����ӷ���ʽ�а�����˳��Ļ�ѧ�������ǣ�1��5��8��1��5��4 |
| D������1 mol ˮʱ��ת��2.5 mol���� |
�����巴Ӧ��mA+nB?eC �¶ȣ�T����ѹǿ��P����ʱ�䣨t���������������ٷ���C%�Ĺ�ϵͼ�����н�����ȷ���ǣ�������


| A��m+n=e |
| B��m+n��e |
| C������Ӧ�����ȷ�Ӧ |
| D��m+n��e |
 ��֪������ȩ��һ�ֻ���ԭ�ϣ�ijʵ��С����������װ�úϳ�����ȩ��
��֪������ȩ��һ�ֻ���ԭ�ϣ�ijʵ��С����������װ�úϳ�����ȩ��
