��Ŀ����
6��ijѧϰС�鰴��ͼʵ�������Ʊ��������������ⶨ������������ֵ������ĺ�������
ʵ�飨һ��������������ȡ
��֪����������������Һ���ܽ�Ƚ�С
ʵ�飨��������������ֵ�IJⶨ
ijͬѧͨ���������Ͽ�֪��
a���к�1g���к��е��������������ص�������mg����Ϊ��ֵ����λ��mg/g��ʾ��
b������������������ϡ����Һ�з�Ӧ���ʽ�����
��ѧϰС�������ͼʵ�鲽��ⶨ������������ֵ��
����1��ȡ10mL�Ҵ�������2-3�η�̪��Һ����0.10mol/LKOH��Һ�μ��������ۺ�ɫ�����ã�
����2����������ƽ��ȡ10.0g��Ʒ�����벽��1�����Ƶ���Һ����������ȫ�ܽ����0.10mol/LKOH��Һ�ζ���ֱ�������ۺ�ɫ��������5s����ɫ��Ϊ�յ㣮
����3���ظ��ⶨ������������������ֵ �ش��������⣺
��1��ʵ�飨һ����Ũ����������Ǵ�������ˮ����
��2����ͼ������2���ʺϵ�װ��B�� ������װ�úͼг�������ʡ�ԣ�

��3��ϴ��-��Һ���ڲ����ڵ�Ŀ����ϴȥ�����в�����CO32-��
��4��ʵ�飨��������1�����Ҵ���Ŀ����Ϊ�ܼ�ʹ���������ͱ�Һ���ܣ�
��5�����в����ᵼ����ֵ�ⶨ���ƫ�ߵ���AB
A װ�������ر�Һ�ĵζ���ˮϴ��ֱ�ӵζ�
B ��Һ�����ۺ�ɫ��������30�벻��ɫ
C ��ƿ������ˮϴ����δ���Tʢ�Ŵ���Һ
D ���ͣ���ƿ������Һ����
��6�����ݼ�¼���±���������ֵ1.12mg/g��
| ���� | �ζ�ǰ����/mL | �ζ������/mL |
| ��1�� | 0.00 | 1.98 |
| ��2�� | 1.98 | 4.00 |
| ��3�� | 4.00 | 5.80 |
| ��4�� | 5.80 | 7.80 |
���� ��1��ʵ�飨һ������ˮ�Ҵ��ͱ�������Ũ����Ĵ������·���������Ӧ��������������ˮ����Ӧ�ǿ��淴Ӧ��Ũ������ˮ�Կ��Դٽ�ƽ��������У�
��2���������������ʵķе㲻ͬ�ӻ�����з����ij�����ʵ�ʵ���������Ҫ�¶ȼƿ��Ʒе㣬ͨ����������������ɷֵõ����ݴ�ѡ��װ�ã�
��3��ϴ��-��Һ���ڲ����ڼ��뱥���Ȼ�����Һʱϴ�����ж����̼������Һ��
��4����������������ˮ��Һ���������Ҵ�������
��5��c�����⣩=$\frac{c������V������}{V�����⣩}$������Һ����仯�ж����ı仯��
��6������ͼ�����ݼ���ƽ�����ı���Һ��������$\frac{c������V������}{V�����⣩}$���㣮
��� �⣺��1��ʵ�飨һ������ˮ�Ҵ��ͱ�������Ũ����Ĵ������·���������Ӧ��������������ˮ����Ӧ�ǿ��淴Ӧ��Ũ������ˮ�Կ��Դٽ�ƽ��������У�Ũ����������Ǵ�������ˮ����
�ʴ�Ϊ����������ˮ����
��2���������������ʵķе㲻ͬ�ӻ�����з����ij�����ʵ�ʵ���������Ҫ�¶ȼƿ��Ʒе㣬ͨ����������������ɷֵõ����ݴ�ѡ��װ��ΪB��
�ʴ�Ϊ��B��
��3���������������뱥��̼������Һ�����Ҵ����к��������������������ֲ㣬���뱥���Ȼ�����Һʱϴ�����ж����̼������Һ��
�ʴ�Ϊ��ϴȥ�����в�����CO32-��
��4��ʵ�飨��������1�����Ҵ���Ŀ����Ϊ�ܼ�ʹ���������ͱ�Һ���ܣ����ڵζ�ʵ����У�
�ʴ�Ϊ����Ϊ�ܼ�ʹ���������ͱ�Һ���ܣ�
��5��A��װ�������ر�Һ�ĵζ���ˮϴ��ֱ�ӵζ�������Һ��Ũ�ȼ�С���ζ�ʵ�������ı���Һ������ⶨ���ƫ�ߣ���A��ȷ��
B����Һ�����ۺ�ɫ��������30�벻��ɫ�����ı���Һ������ⶨ���ƫ�ߣ���B��ȷ��
C����ƿ������ˮϴ����δ���Tʢ�Ŵ���Һ��������Һ���������ʵ������䣬�ⶨ�����Ӱ�죬��C����
D�����ͣ���ƿ������Һ������������Һ��ʧ�����ı���Һ�����С���ⶨ���ƫ�ͣ���D����
�ʴ�Ϊ��AB��
��6��ȡ10mL�Ҵ�������2-3�η�̪��Һ����0.10mol/LKOH��Һ�μ��������ۺ�ɫ�����ã���������ƽ��ȡ10.0g��Ʒ�����벽��1�����Ƶ���Һ����������ȫ�ܽ����0.10mol/LKOH��Һ�ζ���ֱ�������ۺ�ɫ��������5s����ɫ��Ϊ�յ㣬���ı���Һ�������ͼ�����ݷ�������3�βⶨ������ƫ����ȥ�����õ�1�Ρ���2�Ρ���4�μ���ƽ��������Һ���=$\frac{1.98+4.00-1.98+7.80-5.80}{3}$=2.00ml�����������������ʵ���=0.10mol/L��0.002L=0.0002mol����
�к�1g���к��е��������������ص�������mg����Ϊ��ֵ����λ��mg/g��ʾ��������Ʒ����ֵ=$\frac{0.0002mol��56g/mol}{10g}$=0.00112g/g=1.12mg/g��
�ʴ�Ϊ��1.12��
���� ���⿼����������ɡ����ʺ�����ʵ��ⶨ�������ζ�ʵ����̷���ƫ��ʵ���������������ʵ����̵�����Ӧ�õ�֪ʶ�㣬���ջ����ǽ���ؼ�����Ŀ�Ѷ��еȣ�

 ������ϵ�д�
������ϵ�д�| Ԫ�ش��� | A | B | C | D | E | F | G | H |
| ԭ�Ӱ뾶/pm | 37 | 160 | 70 | 66 | 186 | 143 | 104 | 99 |
| ����ϼ� | +1 | +2 | +5 | +1 | +3 | +6 | +7 | |
| ��ͻ��ϼ� | -3 | -2 | -2 | -1 |
��1��G��Ԫ�����ڱ��е�λ���ǵ�������VIA�壨�����ں��壩
��2����������Ԫ���У�����������ˮ����������ǿ����HClO4���ѧʽ������̬�⻯��ˮ��ҺpH��7����NH3���ѧʽ����
��3��B��D��E��F����Ԫ�ص����ӣ������Ӱ뾶������O2-�������ӷ��ţ���
��4��A2D�ĵ���ʽ��
 ��B��H����Ԫ���γɻ�����ĵ���ʽ��
��B��H����Ԫ���γɻ�����ĵ���ʽ�� ��
����5��A��C��H����Ԫ����ɵĻ�����Ļ�ѧʽ��NH4Cl���û������������ӻ��������ӡ����ۡ�����
��6��E��F����Ԫ�ص�����������ˮ�������Ӧ�����ӷ���ʽ��Al��OH��3+OH-=AlO2-+2H2O��
 ����X��������ͺ˴Ź���������������ԭ�ӵĻ�ѧ����ֻ��һ�֣����ݷ���������˵���в���ȷ���ǣ�������
����X��������ͺ˴Ź���������������ԭ�ӵĻ�ѧ����ֻ��һ�֣����ݷ���������˵���в���ȷ���ǣ�������| A�� | X�ķ���ʽΪC5H4 | |
| B�� | X��̼ԭ�ӵĻ�ѧ������2�� | |
| C�� | 1 mol X��һ�������¿���2 mol����������Ӧ | |
| D�� | X����ʹ���Ը��������Һ��ɫ |
 ��ش��������⣮
��ش��������⣮��1��ˮ�ĵ���ƽ��������ͼ��ʾ����A���ʾ25��ʱˮ�ĵ����ƽ��ʱ������Ũ�ȣ�B���ʾ100��ʱˮ�ĵ����ƽ��ʱ������Ũ�ȣ�
��100��ʱ1mol•L-1��NaOH��Һ�У���ˮ�������c��H+��=1��10-12mol•L-1��KW��25�棩��KW��100�棩�����������������=������
��25��ʱ����ˮ�ĵ���ƽ����ϵ�м�������NH4Cl���壬��ˮ�ĵ���ƽ���Ӱ���Ǵٽ�����ٽ����������ơ���Ӱ�족����
��2������ƽ�ⳣ���Ǻ���������ʵ���̶�ǿ����������֪������ݣ�
| ��ѧʽ | ����ƽ�ⳣ����25�棩 |
| HCN | K=4.9��10-10 |
| CH3COOH | K=1.8��10-5 |
| H2CO3 | K1=4.3��10-7��K2=5.6��10-11 |
��25��ʱ����Ũ�ȵ�CH3COOH��Һ��NaOH��Һ�������ϣ���c��Na+����c��CH3COO-�������������������=������
| A�� | 10s-15sC��NH3������������0.25 mol/L | |
| B�� | ��ѧ��Ӧ���ʵĹ�ϵ��3v����H2��=2v����NH3�� | |
| C�� | �ﵽƽ����������NH3��ƽ�������ƶ���v������ | |
| D�� | ����ѹǿ�������Ar���壬v����С |
| A�� | ������������ | B�� | �������������� | ||
| C�� | ������������������ | D�� | ����������������� |
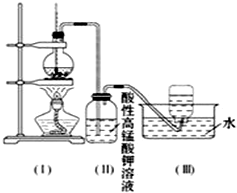 ��ͼ��ʾ����ʵ�����ͨ������Ũ�������Ҵ���ϼ�������ϩ������һ��ʱ�����Һ���к�ɫ������֣���һ��ʱ��������ữ�ĸ��������Һ��ɫ����������֪�������������к���CH2=CH2��SO2��CO2��H2O��
��ͼ��ʾ����ʵ�����ͨ������Ũ�������Ҵ���ϼ�������ϩ������һ��ʱ�����Һ���к�ɫ������֣���һ��ʱ��������ữ�ĸ��������Һ��ɫ����������֪�������������к���CH2=CH2��SO2��CO2��H2O��