��Ŀ����
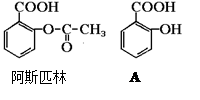
 ҽҩ��˹ƥ�ֵĽṹ��ʽ��ͼ���Իش�
ҽҩ��˹ƥ�ֵĽṹ��ʽ��ͼ���Իش���1����˹ƥ�ֿɿ����������ʣ��ڷ�����θ�������£���˹ƥ�ַ���ˮ�ⷴӦ������A��B���ֲ������A�Ľṹ��ʽ��ͼ����B�Ľṹ��ʽΪ��
CH3COOH
CH3COOH
��2����˹ƥ�ָ�С�մ�NaHCO3��ͬʱ���ã���ʹ����ˮ�����A��С�մ�Ӧ�����ɿ�����������Һ�ų������εĽṹ��ʽΪ��


��3������ˮ�����A��NaOH��Һ��Ӧ�Ļ�ѧ����ʽΪ��


��4������ˮ�����A ��Ũ��ˮ��Ӧ�Ļ�ѧ����ʽΪ��


��������1����˹ƥ�ֺ���������ˮ�������ˮ��������ᣬBΪ���ᣬ�����Ȼ���
��2��AΪˮ���ᣬ�����Ȼ��ͷ��ǻ��������Ȼ�����̼�����Ʒ�Ӧ��
��3��A�к����Ȼ��ͷ��ǻ����������ԣ�����NaOH�����кͷ�Ӧ��
��4��A�к��з��ǻ���������ˮ����ȡ����Ӧ��
��2��AΪˮ���ᣬ�����Ȼ��ͷ��ǻ��������Ȼ�����̼�����Ʒ�Ӧ��
��3��A�к����Ȼ��ͷ��ǻ����������ԣ�����NaOH�����кͷ�Ӧ��
��4��A�к��з��ǻ���������ˮ����ȡ����Ӧ��
����⣺��1����˹ƥ�ֺ���������ˮ������ ��CH3COOH���ʴ�Ϊ��CH3COOH��
��CH3COOH���ʴ�Ϊ��CH3COOH��
��2��AΪˮ���ᣬ�����Ȼ��ͷ��ǻ��������ԣ�-COOH��H2CO3��C6H5OH��HCO3-����A��NaHCO3��Ӧ��������Ϊ ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
��3��A�к����Ȼ��ͷ��ǻ����������ԣ�����NaOH�����кͷ�Ӧ������ʽΪ ��
��
�ʴ�Ϊ�� ��
��
��4��A�к��з��ǻ���������ˮ����ȡ����Ӧ���ǻ���λ�Ͷ�λ��ԭ�ӿɱ�ȡ������Ӧ�ķ���ʽΪ ��
��
�ʴ�Ϊ�� ��
��
 ��CH3COOH���ʴ�Ϊ��CH3COOH��
��CH3COOH���ʴ�Ϊ��CH3COOH����2��AΪˮ���ᣬ�����Ȼ��ͷ��ǻ��������ԣ�-COOH��H2CO3��C6H5OH��HCO3-����A��NaHCO3��Ӧ��������Ϊ
 ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
����3��A�к����Ȼ��ͷ��ǻ����������ԣ�����NaOH�����кͷ�Ӧ������ʽΪ
 ��
���ʴ�Ϊ��
 ��
����4��A�к��з��ǻ���������ˮ����ȡ����Ӧ���ǻ���λ�Ͷ�λ��ԭ�ӿɱ�ȡ������Ӧ�ķ���ʽΪ
 ��
���ʴ�Ϊ��
 ��
�����������⿼���л���Ľṹ�����ʣ��������л�������ŵ����ʵĿ��飬��Ŀ�ѶȲ���ע���л������Ե�ǿ���Ƚϣ��״���Ϊ��2����

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
 ҽҩ��˹ƥ�ֵĽṹ��ʽ���£��Ը��ݰ�˹ƥ�ֵĽṹ�ش�
ҽҩ��˹ƥ�ֵĽṹ��ʽ���£��Ը��ݰ�˹ƥ�ֵĽṹ�ش�
 ��
��


 ҽҩ��˹ƥ�ֵĽṹ��ʽ��ͼ1��ʾ���Ը��ݰ�˹ƥ�ֵĽṹ�ش�
ҽҩ��˹ƥ�ֵĽṹ��ʽ��ͼ1��ʾ���Ը��ݰ�˹ƥ�ֵĽṹ�ش�