��Ŀ����
10����������0.1mol•L-1NaOH��Һ480mL�����ݴˣ��ش��������⣺����������������Һ��Ҫ�õ��IJ�����������Ͳ���ձ�������������ͷ�ιܡ���500mL����ƿ��
��ʵ��ʱ��Ҫ������ƽ������������2.0g��
?����ʱ������ȷ�IJ���˳���ǣ���ĸ��ʾ��ÿ����ĸֻ����һ�Σ�?BCAFED��
A����30mLˮϴ���ձ�2��3�Σ�ϴ��Һ��ע������ƿ
B��ȷ��ȡ���������������ƹ������ձ��У��ټ�������ˮ��Լ30mL�����ò���������������ʹ�����ܽ�
C�����ܽ������������Һ��ȴ���º��ز�����ע������ƿ��
D��������ƿ�ǽ������µߵ�ҡ��
E�����ý�ͷ�ιܼ�ˮ��ʹ��Һ����ǡ����̶�����
F������������ƿ��С�ļ�ˮ��ֱ��Һ��ӽ��̶�1��2cm��
������0.1mol•L-1NaOH��Һ��ʵ���У�����������²������ᵼ��������Һ��Ũ��ƫ�����AC ����д��ĸ����
A������ʱ�������������
B��δϴ���ܽ�NaOH���ձ�
C��NaOH���ձ����ܽ��δ��ȴ������ת�Ƶ�����ƿ��
D������ƿδ���T����������Һ
E������ʱ���ӿ̶���
F�����ݺ�����ƿ������ҡ�ȣ����ú�Һ�治���̶��ߣ��ټ�ˮ���̶��ߣ�
���� ������һ�����ʵ���Ũ����Һһ�㲽�裺���㡢�������ܽ⡢��Һ��ϴ�ӡ����ݡ�ҡ�ȵȣ��ݴ�ѡ����Ҫ����������������Һ���ѡ������ƿ���
������m=CVM������Ҫ���ʵ�������
������һ�����ʵ���Ũ����Һһ�㲽�裺���㡢�������ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȡ�װƿ���ݴ�����
�ܷ������������ʵ����ʵ�������Һ�����Ӱ�죬����C=$\frac{n}{V}$������������
��� �⣺�ټ��㡢�������ܽ⡢��Һ��ϴ�ӡ����ݡ�ҡ�ȵȣ��õ�������������ƽ��ҩ�ס���Ͳ���ձ�������������ͷ�ιܡ�������ƿ������0.1mol•L-1NaOH��Һ480mL��Ӧѡ��500mL����ƿ�����Ի�ȱ�ٵ�������500mL����ƿ��
�ʴ�Ϊ��500mL����ƿ��
������0.1mol/L NaOH��Һ500mL��Ӧ��ȡ������������m=0.1mol/L��0.5L��40g/mol=2.0g��
�ʴ�Ϊ��2.0��
������һ�����ʵ���Ũ����Һһ�㲽�裺���㡢�������ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȡ�װƿ�ȣ�������ȷ��˳��Ϊ��?BCAFED��
�ʴ�Ϊ��?BCAFED��
��A������ʱ������������룬���³�ȡ��������������ƫ�������ʵ����ʵ���ƫ����ҺŨ��ƫ�ߣ���Aѡ��
B��δϴ���ܽ�NaOH���ձ������²���������ģ����ʵ����ʵ���ƫС����ҺŨ��ƫС����B��ѡ��
C��NaOH���ձ����ܽ��δ��ȴ������ת�Ƶ�����ƿ�У�������Һ���ƫС����ҺŨ��ƫ��Cѡ��
D������ƿδ���T����������Һ�������ʵ����ʵ�������Һ�����������Ӱ�죬��ҺŨ�Ȳ��䣬��D��ѡ��
E������ʱ���ӿ̶��ߣ�������Һ���ƫ����ҺŨ��ƫ�ͣ���E��ѡ��
F�����ݺ�����ƿ������ҡ�ȣ����ú�Һ�治���̶��ߣ��ټ�ˮ���̶��ߣ�������Һ���ƫ����ҺŨ��ƫС����F��ѡ��
��ѡ��AC��
���� ���⿼����һ�����ʵ���Ũ����Һ�����ƣ���ȷ����ԭ�������������ǽ���ؼ���ע������ƿ���ѡ��ʹ��ע�������Ŀ�ѶȲ���

 ����ͼ���������������ϵ�д�
����ͼ���������������ϵ�д� ����ѧҵ���Ե�����ϵ�д�
����ѧҵ���Ե�����ϵ�д�| A�� | NaCl���� | B�� | Һ̬�Ȼ��� | C�� | ���ڵ�KNO3 | D�� | NH3•H2O |
| A�� | ����һ�����ʵ���Ũ�ȵ���Һʵ���У�����ʱ�����ӹ۲�̶��ߣ�������������Һ��Ũ��ƫ�� | |
| B�� | ��Һ©�����ϲ���²�Һ������Դ��¿����� | |
| C�� | ������ʹ��ʱ��ˮ�ȿ��Դ��Ͽڽ�Ҳ���Դ��¿ڽ� | |
| D�� | �����ö����ЧӦ����Fe��OH��3�����CuSO4��Һ |
| A�� | ���ڵ�NaCl | B�� | KNO3��Һ | C�� | Cu | D�� | �ƾ� |
| A�� | Cl2 | B�� | SO2 | C�� | NH3 | D�� | O2 |
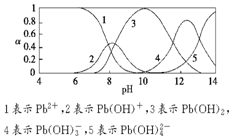 ˮ�����ؽ���Ǧ����Ⱦ���ⱸ�ܹ�ע��ˮ��Һ��Ǧ�Ĵ�����̬��Ҫ��Pb2+��Pb��OH��+��Pb��OH��2���ܣ���Pb��OH��3-��Pb��OH��42-������̬��Ũ�ȷ���������ҺpH�仯�Ĺ�ϵ��ͼ��ʾ��
ˮ�����ؽ���Ǧ����Ⱦ���ⱸ�ܹ�ע��ˮ��Һ��Ǧ�Ĵ�����̬��Ҫ��Pb2+��Pb��OH��+��Pb��OH��2���ܣ���Pb��OH��3-��Pb��OH��42-������̬��Ũ�ȷ���������ҺpH�仯�Ĺ�ϵ��ͼ��ʾ��
 ��
�� ����ӦE-��F�Ļ�ѧ����ʽ�ǣ�
����ӦE-��F�Ļ�ѧ����ʽ�ǣ� $��_{��}^{��������/����Һ}$
$��_{��}^{��������/����Һ}$ +HBr��
+HBr��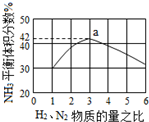 ���ǵ����Ϻ����ḻ��һ��Ԫ�أ������仯�����ڹ�ũҵ������������������Ҫ���ã���������Ҫ�ĵ��ʣ��Dz������Ļ�����Ʒ֮һ���乤ҵ�ϳ�ԭ��Ϊ��N2��g��+3H2��g��?2NH3��g����H=-92.4kJ•mol-1�����ܱ������У�ʹ2mol N2��6mol H2��Ϸ������Ϸ�Ӧ��
���ǵ����Ϻ����ḻ��һ��Ԫ�أ������仯�����ڹ�ũҵ������������������Ҫ���ã���������Ҫ�ĵ��ʣ��Dz������Ļ�����Ʒ֮һ���乤ҵ�ϳ�ԭ��Ϊ��N2��g��+3H2��g��?2NH3��g����H=-92.4kJ•mol-1�����ܱ������У�ʹ2mol N2��6mol H2��Ϸ������Ϸ�Ӧ��