��Ŀ����
10�������������壨FeSO4•7H2O����ҽҩ������Ѫ����ij����С��ⶨ�ò�Ѫ������Ԫ�صĺ�����ʵ�鲽�����£�
��1������ڼ������H2O2��Ŀ���ǽ�Fe2+ȫ������ΪFe3+��
��2��������з�Ӧ�����ӷ���ʽΪFe3++3NH3•H2O=Fe��OH��3��+3NH4+��
��3���������һϵ�д����IJ������裺���ˡ�ϴ�ӡ����ա���ȴ��������
��4��ʵ������Ũ��������250mL1mol/L��ϡ���ᣬ����ʱ�õ��IJ������������ձ����⣬����250mL����ƿ����Ͳ������������ͷ�ιܣ�
��5����ʵ������ģ���ÿƬ��Ѫ������Ԫ�ص�����0.07ag���ú�a�Ĵ���ʽ��ʾ����
���� ������ͼ��֪����ʵ��ԭ��Ϊ����ҩƷ�е�Fe2+�γ���Һ����Fe2+����ΪFe3+��ʹFe3+ת��Ϊ����������������ת��Ϊ��������ͨ���ⶨ�����������������㲹Ѫ������Ԫ�صĺ�����
��1��˫��ˮ���������ԣ������������ܽ�Fe2+ȫ������ΪFe3+��������ͼ��֪������H2O2�ǽ�Fe2+����ΪFe3+��
��2��������ǽ�Fe3+ת��Ϊ��������������
��3���������һϵ�д�������������������Һ����ת��Ϊ����������Ҫ���ˡ�ϴ�ӵ�����������Ȼ��������������������ȴ�������������������
��4������һ�������1mol/L��ϡ���ᣬ����ʱ��Ҫ����������Ͳ���������ձ�����ͷ�ιܣ�����ƿ��
��5��������Ԫ���غ��֪ag����������Ԫ�ص�������Ϊ10Ƭ��Ѫ���������������ݴ˼��㣮
��� �⣺��1��˫��ˮ���������ԣ������������ܽ�Fe2+ȫ������ΪFe3+��
�ʴ�Ϊ����Fe2+ȫ������ΪFe3+��
��2��������ǽ�Fe3+ת��Ϊ����������������Ӧ���ӷ���ʽΪFe3++3NH3•H2O=Fe��OH��3��+3NH4+��
�ʴ�Ϊ��Fe3++3NH3•H2O=Fe��OH��3��+3NH4+��
��3���������һϵ�д�������������������Һ����ת��Ϊ����������Ҫ���ˡ�ϴ�ӵ�����������Ȼ��������������������ȴ�������������������
�ʴ�Ϊ�����ˣ����գ�
��4������һ�������1mol/L��ϡ���ᣬ����ʱ��Ҫ�������в���i�����ձ�����ͷ�ιܡ�250ml����ƿ����Ͳ��
�ʴ�Ϊ��250mL����ƿ����Ͳ������������ͷ�ιܣ�
��5��ag����������Ԫ�ص�������Ϊ10Ƭ��Ѫ������������������ÿƬ��Ѫ������Ԫ�ص�����$\frac{ag��\frac{112}{160}}{10}$��100%=0.07ag��
�ʴ�Ϊ��0.07a��
���� ���⿼��ѧ����ʵ��ԭ����ʵ����������⡢���ʷ����ᴿ��Ԫ�ػ��������ʡ���ѧ����ȣ��Ѷ��еȣ����ʵ��ԭ���ǽ���Ĺؼ�����Ҫѧ���߱���ʵ�Ļ���֪ʶ���ۺ�����֪ʶ�������⡢��������������

 ����С��ʿ���������ϵ�д�
����С��ʿ���������ϵ�д�| A�� | Ba��OH��2 | B�� | NaOH | C�� | BaCl2 | D�� | AgNO3 |
| A�� | 35 | B�� | 37 | C�� | 36 | D�� | 35.5 |
| A�� | 1NA������������ռ�����Ϊ22.4 L | |
| B�� | 2NA��������̼���ӵ�����Ϊ88 g | |
| C�� | 0.1 mol/L ��NaCl��Һ�У�Na+��Cl-��������Ϊ0.2NA | |
| D�� | 17 g NH3������ԭ����ΪNA |
| A�� | ������ | B�� | ������ | C�� | �˵���� | D�� | ���������� |
| A�� | ����������Һ����һ������ˮ��Һ�У��μ�AgNO3������ǡ���ܽ� | |
| B�� | ����Cu��OH��2����Һ����һ����CuSO4��Һ�У���������NaOH��Һ | |
| C�� | 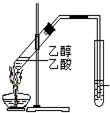 �Ʊ���������������ͼ��ʾ��ʵ��װ�� �Ʊ���������������ͼ��ʾ��ʵ��װ�� | |
| D�� | ����ϩ�ͱ�����������Ȼ�̼��Һ�ֱ�μӵ���������ϩ�ͱ��� |
| A�� | ���������ܶȲ��� | |
| B�� | �����ڵ���ѹǿ����ʱ��仯 | |
| C�� | �����и���ֵ������������ʱ��仯 | |
| D�� | ��λʱ��������2n mol��AB��ͬʱ����n mol��B2 |
| A�� | H2SeO4�������Ա�Cl2�� | B�� | SeO2�������Ա�SO2�� | ||
| C�� | H2SeO4�������Ա�H2SeO3ǿ | D�� | ŨH2SeO4�������Ա�HNO3ǿ |
 H2AsO3-+OH�������ӷ���ʽ��ʾ��������Һ��c��H2AsO3-����c��AsO33-�������������������=������
H2AsO3-+OH�������ӷ���ʽ��ʾ��������Һ��c��H2AsO3-����c��AsO33-�������������������=������