��Ŀ����
�����б�״���µ��������ʣ�
A.11.2LO2��B.6.8gNH3��C.1.204��1024CH4��D.0.5molCO2��
���������ɴ�С��˳��Ϊ________������������ԭ�����ɴ�С��˳����________��������ɴ�С��˳����________��������ܶ��ɴ�С��˳����________��������ţ�
����ͬ������CO��CO2�������ʵ���֮��Ϊ________����������ԭ�Ӹ�����Ϊ________������̼Ԫ�ص�������Ϊ________����ͬ�����µ������Ϊ________��
C��D��A��B C��B��D��A C��A=D��B D��A��B��C 11��7 11��14 11��7 11��7
����������n= =
=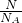 =
=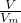 ��Ϸ��ӵĹ��ɺ����ʵ���������㣮
��Ϸ��ӵĹ��ɺ����ʵ���������㣮
��𣺢���n= =
=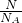 =
=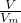 ��֪��
��֪��
A.11.2LO2��n��O2��=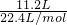 =0.5mol��m��O2��=0.5mol��32g/mol=16g��ԭ����Ϊ2��0.5NA=1NA����=
=0.5mol��m��O2��=0.5mol��32g/mol=16g��ԭ����Ϊ2��0.5NA=1NA����=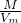 =
=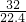 g/L��
g/L��
B.6.8gNH3��n��NH3��=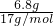 =0.4mol��ԭ����Ϊ4��0.4NA=1.6NA����=
=0.4mol��ԭ����Ϊ4��0.4NA=1.6NA����=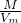 =
= g/L��
g/L��
C.1.204��1024CH4��n��CH4��=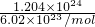 =2mol��m��CH4��=2mol��16g/mol=32g����=
=2mol��m��CH4��=2mol��16g/mol=32g����=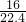 g/L��
g/L��
D.0.5molCO2��m��CO2��=0.5mol��44g/mol=22g��ԭ����Ϊ3��0.5NA=1.5NA����=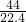 g/L��
g/L��
���ԣ������ɴ�С��˳��ΪC��D��A��B������������ԭ�����ɴ�С��˳����C��B��D��A��������ɴ�С��˳����C��A=D��B��������ܶ��ɴ�С��˳����D��A��B��C��
�ʴ�Ϊ��C��D��A��B��C��B��D��A��C��A=D��B��D��A��B��C��
����CO��CO2��������Ϊmg����n��CO��=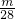 mol��n��CO2��=
mol��n��CO2��= mol��
mol��
�����ʵ���֮��Ϊ��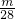 mol��
mol�� mol=11��7��
mol=11��7��
��������ԭ�Ӹ�����Ϊ��11��7��2=11��14��
����̼Ԫ�ص������ȵ���̼Ԫ�ص����ʵ���֮�ȣ�Ϊ��11��7��
��ͬ�����µ������Ϊ�������ʵ���֮�ȣ�Ϊ11��7��
�ʴ�Ϊ��11��7��11��14��11��7��11��7��
���������⿼�����ʵ�������ؼ��㣬��Ŀ�ѶȲ���ע����㹫ʽ�����ã���ǿ�Ը�������⣮
����������n=
 =
=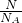 =
=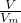 ��Ϸ��ӵĹ��ɺ����ʵ���������㣮
��Ϸ��ӵĹ��ɺ����ʵ���������㣮��𣺢���n=
 =
=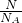 =
=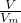 ��֪��
��֪��A.11.2LO2��n��O2��=
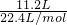 =0.5mol��m��O2��=0.5mol��32g/mol=16g��ԭ����Ϊ2��0.5NA=1NA����=
=0.5mol��m��O2��=0.5mol��32g/mol=16g��ԭ����Ϊ2��0.5NA=1NA����=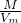 =
=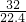 g/L��
g/L��B.6.8gNH3��n��NH3��=
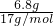 =0.4mol��ԭ����Ϊ4��0.4NA=1.6NA����=
=0.4mol��ԭ����Ϊ4��0.4NA=1.6NA����=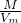 =
= g/L��
g/L��C.1.204��1024CH4��n��CH4��=
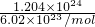 =2mol��m��CH4��=2mol��16g/mol=32g����=
=2mol��m��CH4��=2mol��16g/mol=32g����=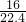 g/L��
g/L��D.0.5molCO2��m��CO2��=0.5mol��44g/mol=22g��ԭ����Ϊ3��0.5NA=1.5NA����=
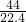 g/L��
g/L�����ԣ������ɴ�С��˳��ΪC��D��A��B������������ԭ�����ɴ�С��˳����C��B��D��A��������ɴ�С��˳����C��A=D��B��������ܶ��ɴ�С��˳����D��A��B��C��
�ʴ�Ϊ��C��D��A��B��C��B��D��A��C��A=D��B��D��A��B��C��
����CO��CO2��������Ϊmg����n��CO��=
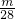 mol��n��CO2��=
mol��n��CO2��= mol��
mol�������ʵ���֮��Ϊ��
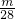 mol��
mol�� mol=11��7��
mol=11��7����������ԭ�Ӹ�����Ϊ��11��7��2=11��14��
����̼Ԫ�ص������ȵ���̼Ԫ�ص����ʵ���֮�ȣ�Ϊ��11��7��
��ͬ�����µ������Ϊ�������ʵ���֮�ȣ�Ϊ11��7��
�ʴ�Ϊ��11��7��11��14��11��7��11��7��
���������⿼�����ʵ�������ؼ��㣬��Ŀ�ѶȲ���ע����㹫ʽ�����ã���ǿ�Ը�������⣮

��ϰ��ϵ�д�
 ����ͼ���������������ϵ�д�
����ͼ���������������ϵ�д�
�����Ŀ