��Ŀ����
��16�֡�����ijѧ����Ƶ��ô���������ȡ����HNO3��Һ��ʵ��װ�ã�����ͼ��ʾ��
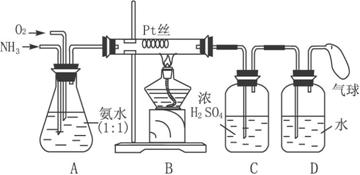
��ش��������⡣
(1)ʵ�����Ʊ�NH3�����з�������ѡ�õ���________(�����)
�ٹ�̬�Ȼ������ʯ�һ�ϼ���
�ڹ�̬�Ȼ�識��ȷֽ�
����ʯ���еμ�Ũ��ˮ
���Ȼ����Һ������������Һ����
(2)װ��B�з�����Ӧ�Ļ�ѧ����ʽΪ______________����ʵ������У�����Pt˿���Ⱥ���ȥ�ƾ��ƣ�����Pt˿���������ֺ��ȣ��ɴ˿��жϸ÷�Ӧ��_____________________��
(3)װ��C��������__________��װ��C�е�������__________��Ϊȷ��D�о����ܶ�����HNO3����ͨ��O2��NH3�������Ӧ����__________��
(4)���ڸ����´�����ʱ���и���Ӧ������4NH3+3O2![]() 2N2+6H2O��������װ���в�����������������D����1.0 mol��L-1��HNO3��Һ150 mL���������ռ��Ļ���������Ϊ400 mL(��״��)������NO2��O2��N2�������Ϊ2��2��1����������NO�İ�ռ���������������Ϊ_________��
2N2+6H2O��������װ���в�����������������D����1.0 mol��L-1��HNO3��Һ150 mL���������ռ��Ļ���������Ϊ400 mL(��״��)������NO2��O2��N2�������Ϊ2��2��1����������NO�İ�ռ���������������Ϊ_________��
��(1)�٢�
![]()
(3)���ջ�������е�NH3 �����ݴ�ŨH2SO3��ð�����Ҽ���ƿ��ŨH2SO4�ϲ��ռ�ʺ���ɫ 2��1
(4)87.5%��![]()
����:
��������(1)��̬NH4Cl������Ȼ�ֽ�ΪNH3��HCl��������dz������½��ΪNH4Cl���ʲ�������NH3������NH3��������ˮ������һ�㲻����Һ�н��С�
(4)1 400 mL����������ʵ���Ϊ��
![]()
����

n(HNO3)=1��0.15 mol=0.15 mol
��������NO�İ�ռ�������İ����������Ϊ��


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�