��Ŀ����
3����ѧ����Ϊ��������һ�ָ�Ч������Ⱦ��������Դ����20����������������Ϊδ���Ķ���ȼ������Դ���о������Ѹ�ٷ�չ����1��Ϊ����Ч��չ��������Դ�����ȱ����Ƶ����۵����������пɹ������ֽϾ�������Դ�ɳ������õ��������ķ�����C����ѡ����ĸ��
A�����ˮB��п��ϡ���ᷴӦC����⺣ˮD���ֽ���Ȼ��
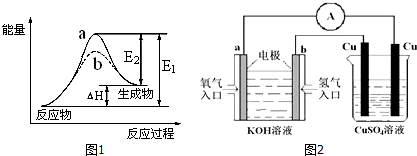
��2����ˮ�ֽ��������������仯��ͼ1��ʾ����ʾʹ�ô���������b���÷�ӦΪ���ȣ����Ȼ������ȣ���Ӧ
��3��1g��������ȫȼ������Һ̬ˮ�ͷų�142.9kJ������д������ȫȼ�յ��Ȼ�ѧ����ʽ��2H2��g��+O2��g��=2H2O��l����H=-571.6kJ•mol-1 �����������𰸾��ɣ���
��4������������CO�ϳɶ����ѵ�������Ӧ���£�
��2H 2��g��+CO��g���TCH 3OH��g������H=-90.8kJ•mol - 1
��2CH 3OH��g���TCH 3OCH 3��g��+H 2O��g������H=-23.5kJ•mol - 1
��CO��g��+H 2O��g���TCO 2��g��+H 2��g������H=-41.3kJ•mol - 1
�ܷ�Ӧ��3H 2��g��+3CO��g���TCH 3OCH 3��g��+CO 2 ��g���ġ�H=-246.4kJ/mol
��5������ȼ�ϵ������ת���ʸߣ����й����ķ�չǰ������������ȼ�ϵ�ؽ���ͼ2��ʾʵ�飺
������ȼ�ϵ���У������ĵ缫��ӦʽΪO2+4e-+2H2O=4OH-��
��ͼ2װ���У�ijһͭ�缫����������6.4g���� a �������ĵ�O 2�ڱ�״���µ����Ϊ1.12L��
��6����������Ѱ����ʵĴ����͵缫���ϣ���N 2��H 2Ϊ�缫��Ӧ���HCl-NH 4ClΪ�������Һ��ȡ����ȼ�ϵ�أ��������缫����ʽN2+8H++6e-�T2NH4+��
���� ��1�������Ͼ�������Դ�ɳ������õ�������������������ԴԽ��Խ�ã�
��2�������ܽ��ͷ�Ӧ��ܣ�����Ӧ������������������������ʱ���÷�Ӧ�Ƿ��ȷ�Ӧ������Ӧ��������С��������������ʱ���÷�Ӧ�����ȷ�Ӧ��
��3��1g���������ʵ���Ϊ0.5mol��1g������ȫȼ������Һ̬ˮ�ͷų�142.9kJ����������2mol������ȫȼ������Һ̬ˮ�ų�����=$\frac{142.9kJ}{0.5mol}$=571.6 kJ���ݴ���д���Ȼ�ѧ��Ӧ����ʽ��
��4���ݸ�˹��������֪���Ȼ�ѧ����ʽ������Ӧ����ֵ���мӼ���������Ŀ���Ȼ�ѧ����ʽ����Ӧ��Ҳ������Ӧ����ֵ���мӼ����ɸ�˹���ɿ�֪���١�2+��+�۵õ���
��5��������ȼ�ϵ���У������������õ����������������ӣ�
��ͭ�缫��������6.4g����ת�Ƶ��ӵ����ʵ���=$\frac{6.4g}{64g/mol}$��2=0.2mol������ת�Ƶ����غ�����������������
��6��ԭ��ص������Ϸ����õ��ӵĻ�ԭ��Ӧ���ݴ���д�缫��Ӧ��
��� �⣺��1�������Ͼ�������Դ�ɳ������õ�������������������ԴԽ��Խ�ã�
A�����ˮ�˷���Դ�����Ը÷������ã��ʲ�ѡ��
B��п��ϡ����۸�ϸߣ������ã����Ը÷������ã��ʲ�ѡ��
C����⺣ˮ��Դ���ĵͣ����ã��÷����ã���ѡ��
D���ֽ���Ȼ���˷���Դ�����Ը÷������ã��ʲ�ѡ��
��ѡC��
��2�������ܽ��ͷ�Ӧ��ܣ�����b����ʹ�ô��������ݷ�Ӧ�����������������Դ�С֪���÷�Ӧ�����ȷ�Ӧ��
�ʴ�Ϊ��b�����ȣ�
��3��1g���������ʵ���Ϊ0.5mol��1g������ȫȼ������Һ̬ˮ�ͷų�142.9kJ����������2mol������ȫȼ������Һ̬ˮ�ų�����=$\frac{142.9kJ}{0.5mol}$=571.6 kJ�����Ȼ�ѧ��Ӧ����ʽΪ��2H2��g��+O2��g��=2H2O��l����H=-571.6 kJ•mol-1 �����������𰸾��ɣ���
�ʴ�Ϊ��2H2��g��+O2��g��=2H2O��l����H=-571.6 kJ•mol-1 �����������𰸾��ɣ���
��4����֪����CO��g��+2H2��g��?CH3OH��g����H=-90.8kJ/mol��
��2CH3OH��g��?CH3OCH3��g��+H2O��g����H=-23.5kJ/mol��
��CO��g��+H2O��g��?CO2��g��+H2��g����H=-41.3kJ/mol��
�ɸ�˹���ɿ�֪���١�2+��+�۵�3CO��g��+3H2��g��?CH3OCH3��g��+CO2��g����H=-246.4kJ/mol��
�ʴ�Ϊ��-246.4kJ/mol��
��5��������ȼ�ϵ���У������������õ����������������ӣ��缫��ӦʽΪO2+4e-+2H2O=4OH-��
�ʴ�Ϊ��O2+4e-+2H2O=4OH-��
��ͭ�缫��������6.4g����ת�Ƶ��ӵ����ʵ���=$\frac{6.4g}{64g/mol}$��2=0.2mol��ͨ�������ĵ缫��ӦʽΪO2+4e-+2H2O=4OH-������ת�Ƶ�����ȵã�
��ͨ���������ΪV��
O2+4e-+2H2O=4OH-
22.4L 4mol
V 0.2mol
$\frac{22.4L}{V}=\frac{4mol}{0.2mol}$��
���V=1.12L��
�ʴ�Ϊ��1.12L��
��6�������ϵ����õ��Ӻ������ӷ�Ӧ����笠����ӣ����缫��ӦΪN2+8H++6e-�T2NH4+��
�ʴ�Ϊ��N2+8H++6e-�T2NH4+��
���� ���⿼����ۺϣ��漰ԭ��غ͵���ԭ����ȼ���Ȼ�ѧ��Ӧ����ʽ����д�����������õ�֪ʶ�㣬����ת�Ƶ�����Ƚ��е��ء�ԭ��ط�Ӧ�ļ��㣬֪�������ܸı��ܣ������ı䷴Ӧ�ȣ���Ŀ�ѶȲ���

| A�� | CO��g�� ��Na2O2��s����Ӧ�ų�509kJ����ʱ������ת����Ϊ6.02��1023 | |
| B�� | 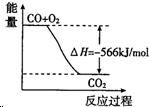 ͼ�ɱ�ʾ��CO����CO2�ķ�Ӧ���̺�������ϵ | |
| C�� | 2Na2O2��s��+2CO2��s���T2Na2CO3��s��+O2��g����H��-452kJ/mol | |
| D�� | CO��ȼ����Ϊ283kJ |
| A�� | ����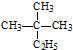 ��ϵͳ����Ϊ2-��-2-�һ����� ��ϵͳ����Ϊ2-��-2-�һ����� | |
| B�� | ��ʯ�ͷ�����Ի��ʯ������ʯ���ѻ��ɻ����ϩ | |
| C�� | ͼ ��ʾ���л������ʽΪC14H12O2���ܷ���������Ӧ ��ʾ���л������ʽΪC14H12O2���ܷ���������Ӧ | |
| D�� | ��ϡ������Һ�У�CH3CO18OC2H5��ˮ�������CH3CO18OH��C2H5OH |

| A�� | H+H��H-H | B�� | H-C1��H+C1 | ||
| C�� | Mg+2HCl�TMgCl2+H2�� | D�� | H2SO4+2NaOH�TNa2SO4+2H2O |
| A�� | H 2O��g���TH 2��g��+O 2��g����H=-485 kJ•mol - 1 | |
| B�� | H 2O��g���TH 2��g��+O 2��g����H=+485 kJ•mol - 1 | |
| C�� | 2H 2��g��+O 2��g���T2H 2O��g����H=+485 kJ•mol - 1 | |
| D�� | 2H 2��g��+O 2��g���T2H 2O��g����H=-485 kJ•mol - 1 |
| A�� | �����¶���ʹ��ѧ��Ӧ����������Ҫԭ���ǽ����˷�Ӧ��� | |
| B�� | ������μӵĻ�ѧ��Ӧ������ѹǿһ����������ѧ��Ӧ���� | |
| C�� | ����Ӧ��Ũ�ȣ�����ߵ�λ����ڻ���ӵİٷ������Ӷ�ʹ��Ч��ײ�������� | |
| D�� | �����ļ�������ߵ�λ����ڻ���Ӱٷ������Ӷ�����ѧ��Ӧ���� |
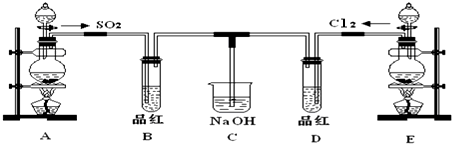
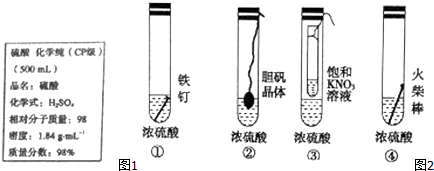
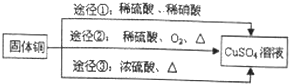
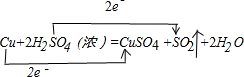 �����л�ԭ����Cu������;��������Ϊ���;���Ǣڣ�ѡ���������ԭ�������ʸߣ�������ȾС��
�����л�ԭ����Cu������;��������Ϊ���;���Ǣڣ�ѡ���������ԭ�������ʸߣ�������ȾС��